
Diffusion, particles of a soluble material spread out
Diffusion is the net movement of particles from a region of high chemical potential to a region of low chemical potential.
"...the irregular movement of the particles produced by the thermal molecular movement"
Albert Einstein, 1905
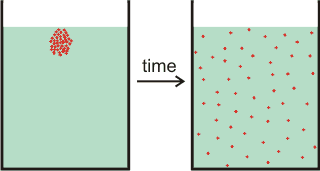
Diffusion b is the net movement of particles (for example, molecules) from a region of high chemical potential (for example, high concentration) to a region of low chemical potential (for example, low concentration) due to random thermal movement, see above right. Such diffusion also involves the movement of water in the opposite direction. The movement is due to the statistical outcome of random Brownian motion and eventually will result in similar concentrations throughout the solution (see the right-hand vessel on the above right).
The diffusive flux (J, the amount of substance moving through a unit area A per unit time t, mol ˣ m-2 ˣ s-1 ) is governed by Fick's first law:
![]()
![]()
where D is the diffusivity (m2 ˣ s-1 ), dφ is the change in concentration (ideally, mol ˣ m-3), and dx is the change in position (m). The diffusion direction is from higher concentration to lower concentration, such that dφ/dx is always negative, and the diffusive flux always positive, in the diffusion direction. Thus, the particle flux is proportional to the concentration gradient.
Einstein defined the diffusivity as
![]()
where r(t) and r(0) are the distances, in 3-dimensional space, to the diffusing particle at time t and at t = 0, respectively.
In a diffusion process, the concentration of a substance in the region of higher concentration gradually decreases, and the concentration of the substance in the region of lower concentration gradually increases. With time, the concentrations gradients dissipate within the bulk. Diffusion increases entropy (randomness), leading to a lower energy state. Eventually, equilibrium is established with a uniform distribution throughout. The concentration change with time is described by Fick's second law:
:
![]()
D is described by the Stokes-Einstein equation for translational diffusion [806], a
![]()
where KB is the Boltzmann constant (J ˣ K-1; kg ˣ m2 ˣ s-2 ˣ K-1), T is the temperature (K), η is the dynamic viscosity (Pa ˣ s; kg ˣ m-1 ˣ s-1), r is the averaged particle radius (m), R is the gas constant (J ˣ mol-1 ˣ K-1; kg ˣ m2 ˣ s-2 ˣ K-1 ˣ mol-1) and N is the Avogadro constant (mol-1; surprisingly, this may be estimated from this simple equation). The 6πηr term comes from Stokes law for the drag of a slow-moving sphere with radius R having a velocity V due to a force F:
F = drag ˣ V
where drag = 6πηr (kg ˣ s-1) ≡ friction on a sphere of radius r, F is the force (N, kg ˣ m ˣ s-2) and V is the particle velocity (m ˣ s-1 ). The drag term includes both the fluid-particle friction (viscous shear stress, 4πηr) and the creation of a pressure difference in the fluid each side of the particle in the direction of flow (2πηr). This simple description of the drag is complicated in liquid water due to the molecular interaction effects [3618].
The slow diffusion within supercooled water has been descibed by a cage-effect where molecules are trapped within transient hydrogen-bonded cages before 'escaping' by jumping into a new environment, so breaking several hydrogen bonds at once [3624].
The diffusion of monatonic and polyatomic ions in aqueous solution have been reviewed [3778]. The diffusion of polyatomic ions is shown to be different from that of monatomic ions due to the rotational self-motion of the former that enhances diffusion in specific cases because of symmetry.
'Diffusion' should not be confused with 'advection' or 'convection'. Advection is the movement due to the velocity of the fluid. Convection applies to the movement of a fluid due, for example, to thermal gradients.
[Back to Top ![]() ]
]
a It has been proposed that this equation should also be associated with the Australian, William Sutherland, who published before Einstein. W. Sutherland, A dynamical theory for non-electrolytes and the molecular mass of albumin, Philosophical Magazine, 6 (1905) 781-785.. [Back]
b The word 'diffusion' is derived from the Latin word 'diffusionem' meaning to "spread out". [Back]
Home | Site Index | Water activity | Brownian motion | Osmotic pressure | LSBU | Top
This page was established in 2006 and last updated by Martin Chaplin on 19 October, 2019