

| Constant | Value |
|---|---|
temperature; zero kelvin (0 K, exactly) |
|
electric current; SI base unit. one A = one C ˣ s-1 = 6.241 509 744 607....ˣ 1018 e- ˣ s-1, based on the exact charge on the electron. |
|
Angular momentum (L) |
L = kg ˣ m2 ˣ s-1 ˣ rad |
| Atomic mass of 12C | 12 g ˣ mol-1 (exactly) |
| Atomic mass unit (u, amu) = Dalton (Da) | 12C atom/12 = 1.660 538 921 ˣ 10-27 kg [934] |
| Atomic unit of action | = reduced Planck's constant |
| Atomic unit of charge | = elementary charge |
| Atomic unit of energy | = Hartree |
| Atomic unit of force = Eh/a0 | 8.238 722 78 ˣ 10-8 N (under revision) |
| Atomic unit of length | = Bohr radius |
| Atomic unit of mass | = electron rest mass in the atomic units system. Not to be confused with the unified atomic mass unit which is the Dalton |
| Atomic unit of pressure = Eh/a03 | 2.942 101 31 ˣ 1013 Pa [934] (under revision) |
| Atomic unit of temperature = Eh/kB | 3.157 775 04 ˣ105 K [934] (under revision) |
| Atomic unit of time = h/2πEh | 2.418 884 326 502 ˣ 10-17 s [934] (under revision) |
| Avogadro constant (NA) | 6.022 140 76 ˣ 1023 mol-1 [2395] (exactly) |
| Avogadro number | dimensionless; 6.022 140 76 ˣ 1023 [2395] |
| Bar (commonly used but not an SI unit) | pressure; 1 bar = 100,000 Pa (exactly) = 0.986 9233 atm = 14.695 9487 7551 psi |
| Barrel (bbl, oil volume; not an SI unit) | = 42 US gallons = 158.987 294 928 L (exactly) |
| Barn (b) | cross-sectional area; 1 b = 100 fm2 = 10-28 m2 |
| Barye (Ba) (not an SI unit) | pressure; 1 Ba = 0.1 Pa (exactly) |
unit of radioactivity; = one nuclear disintegration ˣ s-1, 1 Bq = 27.0270 pCi |
|
number; 1000,000,000; 109 |
|
length; 0.529 177 210 92 ˣ 10-10 m [934] (under revision) |
|
British Thermal Unit (Btu) (not an
SI
unit) |
|
energy; one cal = 4.1868 J (exactly) |
|
Calorie (nutrition only, Cal) |
energy; one Cal = 1 kcal = 4.1868 kJ (exactly) |
| luminous intensity; SI base unit; cd = lm/sr; A lit candle emits light of ≈ 1 cd | |
Celsius zero point (0 °C exactly) |
273.15 K (exactly; one degree Celsius has the same size as one kelvin); |
Charge density |
|
volume; = 0.028 316 846 592 m3 = 28.316 846 592 L (exactly) 1 m3 = 35.314 666 7215 cubic feet |
|
unit of radioactivity, not an SI unit, ; 3.7 ˣ 1010 Bq (exactly) |
|
mass; = atomic mass unit, 12C atom/12 = 1.660 538 921 ˣ 10-27 kg [934] |
|
time: 86,400 seconds (exactly) |
|
Debye (D) (not an
SI
unit) |
|
= mass/volume, kg ˣ m-3 |
|
Dyne (dyn) (not an
SI
unit) |
force; 1 dyn = 10-5 N |
e (base for natural logarithms) |
|
| Electric field strength | V ˣ m-1 ; kg ˣ m ˣ s-3 ˣ A-1 |
Electromagnetic radiation |
|
Enthalpy |
a measure of the heat content of the system; usual units = J ˣ mol-1 |
Entropy |
a measure of disorder and chaos of the system; usual units = J ˣ mol-1 ˣ K-1 |
Erg (erg) (not an
SI
unit) |
energy; 1 erg = 10-7 J |
Electrostatic unit of charge (esu) (not an
SI
unit) |
|
(Fahrenheit temperature) = (Kelvin temperature) ˣ 9⁄5 − 459.67 (Fahrenheit temperature) = (Celsius temperature) ˣ 9⁄5 + 32 One degree Fahrenheit has the same size as 5/9 kelvin and 5/9 °C |
|
| Farad (F) | electric capacitance, = C/V = s4 ˣ A2 ˣ m-2 ˣ kg-1 |
| Faraday | electrical charge: 96,485.332 123 310 0184 C (exactly) |
| Faraday constant (F) | 96,485.332 123 310 0184 C ˣ mol-1 exactly (96,485.332 123 310 0184 J ˣ V-1 ˣ mol-1) [2395]; |
| Foot (ft, ') (not an SI unit) | length; 30.48 cm (exactly) |
| Foot candle (fc) (not an SI unit) | illuminance; 1 fc = lm ˣ ft-2 (1 fc ≈ 10.763 91 lux) |
Gallon (liquid volume; not an
SI
unit)) |
|
RT, Gas constant ˣ temperature (25 °C) |
|
RT/F, Gas constant ˣ temperature/Faraday (25 °C) |
|
| Gauss (G) (not an SI unit) | magnetic flux density; 1 G = 1 Mx ˣ cm-2 = 10-4 T |
Giga anna (Ga, Gyr) (not an
SI
unit) |
time passed; 1000,000,000 years ago |
| Gram molecule | mass; relative molecular mass (molecular weight) in grams = mol |
| Gray (Gy) | Absorbed dose of radiation; Gy = J ˣ kg-1 |
The golden
ratio (φ) |
|
9.806 65 m s-2 (exactly) (≈ 32.1740 ft s-2 ) |
|
frequency (ν), cycles per second: = 1 s-1 (see also terahertz) |
|
Horsepower (hp) (not an
SI
unit) |
power; 746 W (no single definition) |
time; 3600 s |
|
Standard Hydrogen Electrode |
Absolute value 4.44 eV below vacuum; A relative value of 0.00 eV is the standard value. |
Hyperfine splitting frequency of the caesium-133 atom |
Δν(133Cs)hfs is exactly 9,192,631,770 hertz (Hz) (defines the second) |
Inch (in, ") (not an
SI
unit) |
length; 2.54 cm (exactly) |
catalytic activity; one kat = one mol ˣ s-1 |
|
Thermodynamic temperature; SI base unit. It was defined as the fraction 1/273.16 of the thermodynamic temperature of the triple point of set isotopic composition water; (Absolute zero, 0 K = −273.15 °C or −459.67 °F). Now it is defined in terms of the Boltzmann constant, the second, the meter, and the kilogram. 1 K = 0.086 217 38 meV = 0.695 cm1 ( |
|
Kilocalorie (kcal, thermochemical) |
one kcal = 4.1840 kJ (exactly) |
force; 1 kgF = 9.806 65 N (exactly) |
|
work (= energy); 1 kWh = 3.6 MJ (exactly) |
|
| Lightyear | length; one lightyear = 9,460,730,472,580.8 km (exactly) |
| Liter, (L, l; alternatively spelled litre in the UK) | volume; commonly used but not an SI unit; one L = 10-3 ˣ m3 (exactly) |
Ln( ) |
Natural logarithm; = Loge( ). Sometimes referred to as log in the literature |
Loschmidt constant (STP) (= P NA/RT) |
2.651 6462 ˣ 1025 molecules of ideal gas ˣ m-3 (= 44.615 036 mol ˣ m-3) |
| Lumen, (lm) | luminous flux; one lm = one cd ˣ sr; (= cd ˣ m2 ˣ m-2). The luminous efficacy of monochromatic radiation of frequency 540 ˣ 1012 Hz is exactly 683 lm ˣ W-1 |
| Luminance, (Lv) | one Lv = one cd ˣ m-2 |
| Lux, (lx) | illuminance; 1 lx = 1 cd ˣ sr ˣ m-2 |
time passed; 1 Ma = 1,000,000 years ago |
|
| Maxwell (Mx) (not an SI unit) | magnetic flux; 1 Mx = 1 G ˣ cm2 = 10-8 Wb |
| Meter (m); alternatively spelled metre in the UK | length; SI base unit. defined by the speed of light below |
| Micron (µ, µm), micrometer | length: = 1 µm = 10-6 m |
| Mil (not an SI unit) | length; easily confused as may mean either, m ˣ 10-3 or in ˣ 10-3 |
| Mile (international, not an SI unit) | length: 1,609.344 m (exactly) |
| Minute | (min) time; 1 min = 60 s ('), plane angle; 1' = π/10800 rad (a circle |
mm-Hg (not an
SI
unit) |
|
1 m = 1 mol ˣ (kg solvent)-1 (see for conversion to molarity) |
|
Molality, standard (m°) |
m° = 1 mol ˣ (kg solvent)-1 |
Molar concentration (M) |
1 mol ˣ (L solution)-1 = 103 mol ˣ m-3 (see for conversion to molality) |
| Molar volume of ideal gas (STP) | = R ˣ 273.15/100,000 m3 ˣ mol-1 = 22.710 953 L ˣ mol-1 (22.414 L ˣ mol-1 at 101.325 kPa) |
| Mole (mol) | amount; SI base unit. an Avogadro number of items |
| Mya (not an SI unit) | time passed; 1 Mya = 1,000,000 years ago |
| Nernst constant, = RT/F | 25.692576 mV (25 °C); Loge(10) ˣ RT/F = 0.059 159 243 V (25 °C) |
| Newton (N) | force; N = 1 kg ˣ m ˣ s-2 |
| Oersted (Oe) | magnetic field; 1 Oe |
| Ohm (Ω) | electric resistance; = V/A, 1 Ω = 1 kg ˣ m2 ˣ s-3 ˣ A-2 |
| Osmole (Osm) (not an SI unit) | amount; the number of moles of solute that contribute to the osmotic pressure of a solution. Ideally, 1 mole of NaCl in 1 Kg water is 2 Osm ˣ kg-1-water |
| Pascal (Pa) | pressure, stress, 1 Pa = 1 N ˣ m-2 = 1 kg ˣ m-1 ˣ s-2 |
| Parts per billion (ppb) | ppb = parts per 1000,000,000; for example, 1 µg ˣ kg-1 |
| Parts per million (ppm) | ppm = parts per 1000,000; for example, 1 mg ˣ kg-1; also used in NMR |
Permeability |
the degree of magnetization of a material in response to a magnetic field = H ˣ m-1 |
Permittivity |
the ability of a substance to store electrical energy in an electric field = F ˣ m-1 |
Pint (liquid volume; not an SI unit) |
|
Poise (P) (not an
SI
unit) |
|
Pound (lb) (avoirdupois, US and UK) (not an SI unit) |
mass = 0.453 592 37 kg (exactly) |
Pound weight (lbf)
(not an
SI
unit) |
force = 4.448222 N |
Proton charge |
(+ve) 1.602 176 634 ˣ 10-19 C (exactly) |
Proton gyromagnetic ratio (γP) |
|
psu (practical salinity unit) (not an
SI
unit) |
The psu has no dimensional units; see elsewhere |
Quadrillion |
number; 1000,000,000,000,000; 1015 |
| Radiance | W ˣ sr-1 ˣ m-2 ; kg ˣ s-3 |
| Second (s) | time; SI base unit. The second is equal to the duration of exactly 9,192,631,770 periods of the radiation corresponding to the transition between the two hyperfine levels of the unperturbed ground state of the 133Cs atom. ΔνCs = 9,192,631,770 s-1 exactly. This definition of the second may be updated in 2026. |
| Second ('') | (''), Plane angle; 1'' = π/648,000 rad (a circle |
| Siemens (S) | electric conductance; = A/V, 1 S = 1/Ω = 1 s3 ˣ A2 ˣ kg-1 ˣ m-2 |
| Sievert (Sv) | radiation dose equivalent; Sv = J ˣ kg-1 |
energy ˣ mol-1 ˣ K-1; = kg ˣ m2 ˣ s-2 ˣ mol-1 ˣ K-1 |
|
Spectral luminous efficiency (Kλ) |
|
299,792,458 m ˣ s-1 (exactly, also defines the meter)
[934] |
|
101.325 kPa, 273.15 K, concentration 1 mol ˣ L-1 (1000 mol ˣ m-3) |
|
100 kPa, 273.15 K (exactly) IUPAC |
|
electrical charge; see esu |
|
Standard state (thermodynamic) |
varies as stated, but often; pressure, 100 kPa; temperature, 25.00 °C |
The solid angle of the sphere surface (area A, radius r) from the center, = (A/r2 ) ˣ sr 4π ˣ sr = entire surface of a sphere radius r. The units of sr are m2 ˣ m-2 but usually treated as dimensionless;. |
|
Stokes (St) (not an
SI
unit) |
kinematic viscosity; 1 St = 1 cm2 ˣ s-1 = 10-4 m2 ˣ s-1 |
Sun |
solar irradiation; 1 kW ˣ m-2 |
| Surface tension | N ˣ m-1 ; kg ˣ s-2 |
frequency in cycles per second:one THz = 1012 s-1 = 2π ˣ 1012 radian ˣ s-1 = one ps-1 |
|
Thermal energy (kT at 25 °C) |
|
| Ton (metric ton) (tonne, t) | mass; = 103 kg (1 Mg) |
| Ton (UK; long ton) | mass; = 2,240 lb avoirdupois, UK; = 1016.046 908 80 kg (exactly) |
| Ton (US; short ton) | mass; = 2,000 lb avoirdupois, US; = 907.184 740 kg (exactly) |
| Torr (not an SI unit) | historical pressure unit; one torr = 101325⁄760 Pa (exactly; ≈ 133.322 368 4211 Pa ) ≡ 1 mm Hg |
number; 1000,000,000,000; 1012 |
|
deci- d 10-1;centi- c 10-2; milli- m 10-3; micro- µ 10-6; nano- n 10-9; pico- p 10-12; femto- f 10-15; atto- a 10-18; zepto- z 10-21; yocto- y 10-24; ronto- r 10-27 a; quecto- q 10-30 a deca- da 101; hecto- h 102; kilo- k 103; mega- M 106; giga- G 109; tera- T 1012; peta- P 1015; exa- E 1018; zetta- Z 1021; yotta- Y 1024; ronna- R 1027 a; quecca- Q 1030 a |
|
| Vacuum permeability | |
| Viscosity, dynamic (η) | Pa ˣ s ( kg ˣ m-1 ˣ s-1); 10 poise = 1 Pa ˣ s |
| Viscosity, kinematic (ν) | = η/ρ (m2 ˣ s-1) = dynamic viscosity divided by the density of the liquid; 10,000 stokes = 1 m2 ˣ s-1 |
| Volt (V) | electrical potential; V = amps ˣ ohms (A ˣ Ω); V = power/current (W/A); V = energy/amount of charge (J/C); one V = one kg ˣ m2 ˣ s-3 ˣ A-1 |
| Wavelength (nm) | one nm = 107/wavenumber (cm-1) (electromagnetic; one nm |
| Wavenumber (m-1) (=1/wavelength) | one m-1 = 109/wavelength (nm), one m-1 1000 cm-1 (one cm-1 |
| Wavenumber, wavelength. frequency conversions |
|
| Watt (W) | power, radiant flux; one W = one J ˣ s-1 = one kg ˣ m2 ˣ s-3 |
| Weber (Wb) | magnetic flux; = T ˣ m2 = V ˣ s; 1 Wb = 1 kg ˣ m2 ˣ s-2 ˣ A-1 |
| Year (a, yr) | One Gregorian year = 365.2425 days = 31,556,952 seconds One Julian year = 365.25 days = 31,557,600 seconds One mean Tropical year = 365.2422 days = 31,556,926 seconds |
The relationship between the SI units,
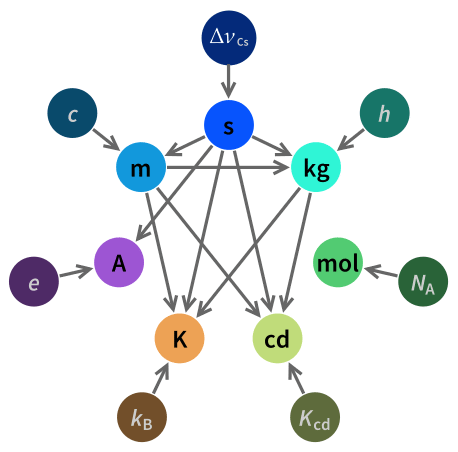
Note that the SI unit definitions [1031] came into force on 20 May 2019 (see right).
s - defined using the frequency of the cesium hyperfine transition (ΔvCs),
kg - defined using the Planck constant (h),
mol -defined using the Avogadro constant (NA ),
cd - defined using the sensitivity of the human eye (luminous efficacy, Kcd),
K - defined using the Boltzmann constant (kB),
A - defined using the elementary electric charge (e),
m - defined using the speed of light (c) .
Generated from a Mathematica notebook at https://github.com/episanty/SI-unit-relations.
[Back to Top ![]() ]
]
a Prefixes awaiting approval, D. Adam, Metric prefixes sought for extreme numbers, Science, 363 (2019) 681. [Back]
Home | Site Index | Water data (156 KB) | Short H2O properties listing | Other data | Further data | LSBU | Top
This page was established in 2007 and last updated by Martin Chaplin on 1 November, 2019