
Glacier ice (1h)

Water has many solid phases (ices), given with their properties below.
![]() Phase diagram
Phase diagram
![]() Ice crystal data
Ice crystal data
![]() Known ices
Known ices
![]() Computer ices (ice 0, ice χ)
Computer ices (ice 0, ice χ)
![]() Vonnegut's ice-nine
Vonnegut's ice-nine
![]() The 'ice-rules'
The 'ice-rules'
The typical tetrahedral arrangement of hydrogen bonds
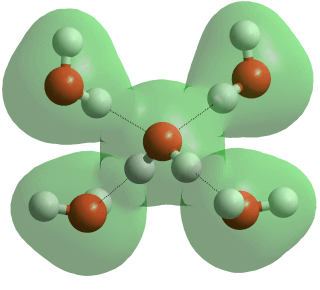
There are eighteen or so crystalline phases [3500] (where the oxygen atoms are in fixed positions relative to each other, but the hydrogen atoms may or may not be disordered, and three amorphous (non-crystalline) phases (see [2145, 2349] for recent reviews of ice research). This large number is due to the open tetrahedrally arranged water molecular structure of hexagonal ice under normal atmospheric pressure and the large number of possible crystal structures that this ice can form as it is progressively crushed under high pressure.
All the crystalline phases of ice involve the water molecules being hydrogen-bonded to four neighboring water molecules (see left, and [1300] for a recent review). In most cases, the two hydrogen atoms are equivalent with the hydrogen bonds of similar strength. The water molecules retain their symmetry obey the 'ice rules' j. For the most part, the ordering of the protons (in fixed positions with lower entropy) occurs at lower temperatures, whereas pressure reduces the distances between second shell neighbors (lower volume and greater van der Waals effects). The H-O-H angle in the ice phases is expected to be a little less than the tetrahedral angle (109.47°), at about 107°. The Clausius Clapeyron equation n for many ice phase changes has to be adapted due to water's negative expansion coefficient and anomalous change in entropy with volume [1147c].
Ice polymorph |
Density, |
Protons f | Crystal h | Symmetry | Dielectric constant, εS i | Notes | ||
|---|---|---|---|---|---|---|---|---|
| Ih, Hexagonal ice | 0.92 |
0.926 | disordered | Hexagonal | P63/mmc | one C6 | 97.5 | |
| Ic, Cubic ice | 0.93 q |
0.933 | disordered | Cubic | four C3 | |||
Isd, Stacking dis-ordered ice |
0.93 |
0.933 | disordered | Trigonal | P3m1 | one C3 | ||
| LDA, Ia b | 0.925 q |
disordered | Non-crystalline | As prepared, may be mixtures of several types | ||||
| HDA c | 1.17 |
disordered | Non-crystalline | As prepared, may be mixtures of several types | ||||
| VHDA d | 1.25 |
disordered | Non-crystalline | |||||
| II, Ice-two | 1.17 |
1.195 | ordered | Rhombohedral | one C3 | 3.66 | ||
| III, Ice-three | 1.14 |
1.160 | disordered | Tetragonal | P41212 | one C4 | 117 | protons may be partially ordered |
| IV, Ice-four | 1.27 |
1.275 | disordered | Rhombohedral | one C3 | metastable in ice V phase space, two interpenetrating ice frameworks | ||
| V, Ice-five | 1.23 |
1.233 | disordered | Monoclinic | C2/c | one C2 | 144 | protons may be partially ordered |
| VI, Ice-six | 1.31 |
1.314 | disordered | Tetragonal e | P42/nmc | one C4 | 193 | two interpenetrating frameworks |
| VII, Ice-seven | 1.50 |
1.591 | disordered | Cubic e | four C3 | 150 | two interpenetrating ice Ic frameworks | |
| VIII, Ice-eight | 1.46 |
1.885 | ordered | Tetragonal e | I41/amd | one C4 | 4 | low-temperature form of ice VII |
| IX, Ice-nine | 1.16 |
1.160 | ordered | Tetragonal | P41212 | one C4 | 3.74 | low-temperature form of ice III, metastable in ice II space |
| X, Ice-ten | 2.51 |
2.785 | symmetric | Cubic e | four C3 | symmetric proton form of ice VII | ||
| XI, Ice-eleven | 0.92 |
0.930 | ordered | Orthorhombic | Cmc21 | three C2 | ordered form of ice Ih phase (needs OH-) | |
| XI, Ice-eleven k | >2.51 |
symmetric | Orthorhombic e | PBcm | distorted | Superionic | ||
| Metallic [1818] k |
|
≈ 12 o | symmetric, O-H-O bent |
Monoclinic e | C2∕m | Superionic | ||
| XII, Ice-twelve | 1.29 |
1.301 | disordered | Tetragonal | one C4 | metastable in ice V phase space | ||
| XIII, Ice-thirteen | 1.23 |
1.247 | ordered | Monoclinic | P21/a | one C2 | ordered form of ice V phase (needs H+) | |
| XIV, Ice-fourteen | 1.29 |
1.294 | mostly ordered | Orthorhombic | P212121 | one C4 | ordered form of ice XII phase (needs H+) | |
| XV, Ice-fifteen | 1.30 |
1.328 | ordered | Pseudo-orthorhombic | one C4 | ordered form of ice VI phase (needs H+) | ||
| XVI, Ice-sixteen | 0.81 |
disordered | Cubic | Fd3m | empty SII clathrate | |||
| Empty clathrate SIII r | 0.59 |
disordered | Cubic | three C4 | empty SIII clathrate | |||
| XVII, Ice-seventeen |
|
0.85 | disordered | Hexagonal | P6122 | one C6 | empty, was H2-filled ice | |
| XVIII, Ice-eighteen |
|
~3 | liquid | Cubic | Fm3m | four C3 three C4 |
Superionic; fcc oxygens |
|
Ice Ih may be metastable with respect to empty clathrate structures (Ice-sixteen, [2252 and Ice-seventeen [2796]]) of lower density under negative pressure conditions (that is, stretched) at very low temperatures [520]. Different research groups have described two different forms of ice-eleven; (a) the high-pressure form (also known as ice-thirteen) involves hydrogen atoms equally-spaced between the oxygen atoms [84] (like ice-ten) in a distorted hexagonal close-packed structure whereas (b) the lower pressure, low temperature, form uses the incorporation of hydroxide defect doping (and interstitial K+ ions) to order the hydrogen-bonding of ice Ih [207], that otherwise occurs too slowly. Another ice-ten has been described, being the proton-ordered form of ice-six (VI); this is now known as ice fifteen. Only hexagonal ice-one (Ih), ice-three (III), ice-five (V), ice-six (VI), ice-seven (VII) and, perhaps, ice-ten (X) can be in equilibrium with liquid water (ice-ten with supercritical water), whereas all the others ices, including ice-two (II, [273]), are not stable in its presence under any conditions of temperature and pressure. The low-temperature ices, ice-two, ice-eight (VIII), ice-nine (IX), ice-eleven (low pressure form), ice-thirteen (XIII) [1002], ice-fourteen (XIV) [1002] and ice fifteen (XV) [1582] all possess (ice-nine and ice-fourteen incompletely) low entropy ordered hydrogen-bonding whereas in the other ices (except ice-ten [80] and ice-eleven where the hydrogen atoms are symmetrically placed and molecules of H2O do not have individual existence) the hydrogen-bonding is disordered even down to 0 K, where reachable; these include all the ices that share a phase boundary with liquid water. Their zero-point energies have been described [3780]. Disordered hydrogen-bonding causes positional disorder in the oxygen atoms of several pm around their crystallographic sites. Also, the disordered ices I, IV, V, VI, and XII, also show glass transitions at low temperatures [2601], associated with the unfreezing of the reorientation dynamics. Ice-four (IV) and ice-twelve (XII) [82] are both metastable within the ice-five phase space. Cubic ice (Ic) is metastable with respect to hexagonal ice (Ih). Ice-seven (VII) undergoes X-ray-induced (≈ 9.7 keV) dissociation to an O2 - H2 alloy g at high pressure (>2.5 GPa) but reverts to ice-seven near its melting point at 700 K and 15 GPa [1383]. A new ice phase has been reported to lie on what had been thought to be the liquid (supercritical) side of ice-seven at high pressures, with approximate triple points of about 700 K, 20 GPa with liquid (supercritical) water and ice-seven and about 1500 K, 40 GPa with liquid (supercritical) and ice-ten [1521]. This may be a plastic phase where only molecular rotations are allowed [2078].
Ice polymorph |
Molecular
environments |
Small
ring size(s)p |
Helix |
Approximate
O-O-O angles, ° |
Ring
penetration hole size |
|---|---|---|---|---|---|
| Ih, Hexagonal ice | 1 |
6 |
None |
All 109.47±0.16 |
None |
| Ic, Cubic ice | 1 |
6 |
None |
109.47 |
None |
| Isd, Stacking dis-ordered ice | 1 |
6 |
None |
109.47 |
None |
| LDA, Ia b | 3+ |
5(9), 6(55) |
None |
mainly
108, 109 and 111 |
None |
| HDA c | 6+ |
5(9), 6(55) |
None |
broad
range |
None |
| VHDA d | 6+ |
5(9), 6(55) |
None |
broad
range |
None [747] |
| II, Ice-two | 2 (1:1) |
6(7), 8(9),10(15) |
None |
80,100,107,118,124,128; 86,87,114,116,128,130 |
None |
| III, Ice-three | 2 (1:2) |
5(1), 7(1), 8(1) |
4—fold |
(1) 91,95,112,112,125,125 (2) 98,98,102,106,114,135 |
None |
| IV, Ice-four | 2 (1:3) |
6(7), 8(18),10(42) |
None |
(1) 92,92,92,124,124,124 (3) 88,90,113,119,123,128 |
some 6 |
| V, Ice-five | 4 (1:2:2:2) |
4(2), 5(3),
6(2), 8(3),9(2),10(12),12(1) |
None |
(1) 82,82,102,131,131,131 (2) 88,91,109,114,118,128 (3) 85,91,101,103,130,135 (4) 84,93,95,123,125,126 |
8 (1 bond) |
| VI, Ice-six | 2 (1:4) |
4(5), 8(9) |
None |
(1) 77,77,128,128,128,128 (2) 78,89,89,128,128,128 |
8 (2 bonds) |
| VII, Ice-seven | 1 |
6 |
None |
109.47 |
every
6 |
| VIII, Ice-eight | 1 |
6 |
None |
109.47 |
every
6 |
| IX, Ice-nine | 2 (1:2) |
5(1), 7(1), 8(1) |
4—fold |
(1) 91,95,112,112,125,125 (2) 98,98,102,106,114,135 |
None |
| X, Ice-ten | 1 |
6 |
None |
109.47 |
every
6 |
| XI, Ice-eleven | 1 |
6 |
None |
109.47 |
None |
| XI, Ice-eleven k | undetermined |
6/4 |
None |
undetermined |
every
6 |
| XII, Ice-twelve | 2 (1:2) |
7(2), 8(3) |
5—fold |
(1) 107,107,107,107,115,115 (2) 67,83,93,106,117,132 |
None |
| XIII, Ice-thirteen | 7 (all equal) |
4(2), 5(3),
6(2), 8(3),9(2),10(12),12(1) |
None |
(1) 82,82,102,131,131,131 (2) 88,91,109,114,118,128 (3) 85,91,101,103,130,135 (4) 84,93,95,123,125,126 |
8 (1 bond) |
| XIV, Ice-fourteen | 2 (1:2) |
7(2), 8(3) |
5—fold |
(1) 107,107,107,107,115,115 (2) 67,83,93,106,117,132 |
None |
| XV, Ice-fifteen | 2 (1:4) |
4(5), 8(9) |
None |
(1) 77,77,122,122,134,134 (2) 87,90,94,124,129,135 |
8 (2 bonds) |
| XVI, Ice-sixteen | 4 (6:6:4:1) |
5(9), 6(1) |
None |
106, 108, 109, 120 |
None |
| Empty clathrate SIII r | 2 (1:3) |
4(12), 6(8), 8(6) |
None |
90, 120, 135 |
None |
| XVII, Ice-seventeen | 1 |
5 |
6-fold |
103, 106, 111, 125 |
None |
| XVIII, Ice-eighteen | 2 |
n/a |
n/a |
90, 180 |
n/a |
n/a = not applicable
The thermal conductivities properties of crystalline and amorphous ices have been reviewed [1202]. The OD/OH Raman stretch bands for D2O/H2O ices have been analyzed and compared [3508]. The vibrational amplitudes and the degree of the phonon localization in nineteen ice forms, both crystalline and amorphous, have been determined by a quasi-harmonic approximation with a classical intermolecular interaction model for water [3783b]. The amplitude in the low pressure ices was found to increase with compression, while the opposite trend was observed in the medium and high pressure ices. The mean square displacements of the ices' oxygen and hydrogen atoms were determined against temperature for ice Ih (O 0.107 ˣ Å-2; H 0.129 ˣ Å-2 at 200 K), LDA (O 0.105 ˣ Å-2; H 0.129 ˣ Å-2 at 200 K) and HDA at 100 MPa (H 0.131 ˣ Å-2 at 200 K), ice III at 300 MPa (O 0.095 ˣ Å-2; H 0.130 ˣ Å-2 at 200 K), VI at 1 GPa (O 0.054 ˣ Å-2; H 0.083 ˣ Å-2 at 200 K), and ice VII at 7 GPa (O 0.032 ˣ Å-2; H 0.058 ˣ Å-2 at 200 K) by a quasi-harmonic approximation method [3783b].
Other stable or metastable phases of ice have been proposed (for example, Ice XIII and ice XIV were proposed earlier than their discovery [958]) but their structures were not established. Several new phases (for example ice i, 'Hexagonal Bilayer Water' and 'Pleated Sheet Water', [1985]) have only been found (so far) in modeling studies, but other ices have been found at confined surfaces. 'Metallic' water, where electrons are freed to move extensively throughout the material and the atoms of water exist as ions, probably exists as an antifluorite type structure m above 1.76 TPa [1138]. It is not thought that any other phases are stable at higher pressures than this.
The proposed topology of the transformations between ice XI ![]() ice II
ice II![]() ice IX, and ice VIII
ice IX, and ice VIII ![]() ice X has been described [1237]. The mechanism of ice crystal formation has been investigated by molecular dynamics by overcoming its timescale limitation. It was suggested that the formation of each ice crystal occurred via high-density water with a similar structure to the formed ice crystal [3908]. [Back to Top
ice X has been described [1237]. The mechanism of ice crystal formation has been investigated by molecular dynamics by overcoming its timescale limitation. It was suggested that the formation of each ice crystal occurred via high-density water with a similar structure to the formed ice crystal [3908]. [Back to Top ![]() ]
]
There are many other possible crystalline structures of solid water (ice) that fit with the tetrahedrality of water's hydrogen-bonding, and that obey the ice rules. These 'metastable' states may be generated using molecular models but whether they are important in the real world needs to be determined by experiment. One such ice is ice 0 (see below), a tetragonal structure (unit cell 12 molecules; 90º, 90º, 90º, 5.93 Å, 5.93 Å, 10.74 Å; 0.95 g ˣ cm-3) containing 5-, 6- and 7-membered rings that has been proposed as a structure formed during the crystallization of ice Ic and ice Ih from supercooled water [2149]. Interestingly this ice 0 structure contains partial dodecahedral clusters consisting of three linked pentamers (H2O)11 as thought to exist in supercooled water and ES.
Ice 0 [2149]; 3 x 3 x3 unit cells viewed down the x- and z-axes. The view down the y-axis is similar to that down the x-axis![Ice 0 [2149]; 3 x 3 x3 unitcells viewed down the x- and z-axes. The view down the y-axis is similar to that down the x-axis Ice 0 [2149]; 3 x 3 x3 unitcells viewed down the x- and z-axes. The view down the y-axis is similar to that down the x-axis](images/ice_0.gif)
In these diagrams of ice 0, the hydrogen-bonding is shown ordered whereas, in reality, it is random, obeying the ice rules.
Interactive structures of ice 0 (Jmol) are available. Another computer ice has been proposed as a metastable link in the crystallization of ice VII at 10 GPa, 425 K [2163].
The empty clathrate S-III ice has been proposed to be the most stable ice phase at very high negative (i.e., very stretched conditions) pressures [2507].
Ice χ (ice-chi, [3589]). Using free-energy computations a further high density (1.272 g ˣ cm-3) ice is found under high external electric field (2.3 V ˣ nm-1) as the most stable structure in the high-pressure/low-temperature region, located between ice II and ice VI, and the low-temperature neighbor of ice V exhibiting two triple points at 606 MPa, 131.23 K (ice II ice V, ice χ) and 945 MPa, 144.24 K (ice V, ice χ, ice VI). The computed ferroelectric crystal structure is orthorhombic with space group Fdd2 with the lattice parameters of the 56-water molecule unit cell being a = 24.34 Å, b = 12.53 Å, and c = 4.32 Å. All water molecules are oriented in the direction of the external electric field. [3589].

Kurt Vonnegut's highly entertaining story concerning an (imaginary) ice-nine, which was capable of crystallizing all the water in the world [83], fortunately, has no scientific basis (see also IE). Ice-nine, in reality, is a proton-ordered form of ice-three, and only exists at very low temperatures and high pressures and cannot exist alongside liquid water under any conditions.
a Left column: experimental density at atmospheric pressure but at the temperature of stability (this will contain crystal boundaries and faults); right column: crystallographic density [1717]. [Back]
b Low-density amorphous ice (LDA). The structural data in the Table is given assuming LDA has the structure of ES. [Back]
c High-density amorphous ice (HDA). The structural data in the Table is given assuming HDA has the structure of crushed CS. [Back]
d Very high-density amorphous ice (VHDA). The structural data in the Table assumes no hydrogen bond rearrangements from LDA or HDA. As VHDA is likely to be a relaxed form of HDA, this assumption seems unlikely [935]. [Back]
e Structure consists of two interpenetrating frameworks. [Back]
f Although primarily ordered or disordered, ordered arrangements of hydrogen-bonding may not be perfect and disordered arrangements of hydrogen-bonding are not totally random as there are correlated and non-bonded preferential effects. [Back]
g This ice is reported to be more likely a trigonal structure made up of 2H3Oδ++ O2δ- + H2 rather than a 2H2 + O2 alloy [1419]. [Back]
h Crystal cell parameters have been collated. The right-hand column gives the space group. [Back]
i Relative permittivities (dielectric constants) fall into two categories depending on whether the hydrogen bonds are ordered (low values) or disordered (high values). [Back]
An H2O ice molecule (a)
obeying the 'ice rules'
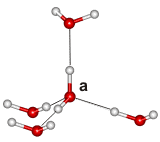
j The 'ice rules': [3844] (also called the Bernal–Fowler rules [766]) Each water molecule (right labeled 'a') has four hydrogen-bonded neighbors, two hydrogen atoms near each oxygen (≈ 1 Å), one hydrogen atom on each O····O bond; thus H-O-H···OH2 and H2O···H-O-H are allowed, but H-O-H···H-O-H and H2O···OH2 are not; see H2O molecule a right). As the H-O-H angles are about 106.6º [717], the hydrogen bonds are not straight (although shown so in the figures). Weaknesses (Bjerrum defects; 66 kJ ˣ mol-1 [3220]) in the ice crystal are apparent where the ice rules are disobeyed. Both O····O contacts, without an intervening proton (L defect, 'leer' defect) and O-H····H-O contacts (D defect, 'doppelt' defect, with two protons between the pair of oxygen atoms) may occur due to molecular rotations where neighboring water molecules fail to adjust their hydrogen-bonding. Another type of defect is the ionic defect caused by the presence of H3O+ and OH- ions (135 kJ ˣ mol-1 [3220]). The addition of ammonium fluoride as a hydrogen-disordering agent in ordered hydrogen bond ices (e.g. ice II and ice VIII) can destroy the hydrogen bond ordering as F- can accept up to 4 H-bonds and NH4+ can donate up to 4 H-bonds and both ions can fit in an ice hydrogen-bonded lattice [3608]). [Back]
k Ice XI was first described as a fully-ionic antifluorite structure formed at around 100 GPa [2539]. Ice XI is also known as ice XIII. These structures have not been experimentally verified and, therefore perhaps, are best not referred to with the numerical designations first used. [Back]
m The antifluorite structure consists of a face-centered cubic (FCC) unit cell with oxygen anions occupying the FCC lattice points (corners and faces), and hydrogen cations occupy the eight tetrahedral sites within the FCC lattice. [Back]
n The Clausius Clapeyron equation can be stated as dT/dP=TΔV/ΔH=ΔV/ΔS where P, T, H, V, and S are the pressure, temperature, enthalpy, volume, and entropy. This may be extended to be
dT/dP=T(sign α2V2 - sign α1V1)ΔV/ΔH
where α represents the thermal expansion coefficients, for use with phases with negative expansion coefficients including the ice phase changes
LDA![]() Ic, HDA
Ic, HDA![]() LDA, LDA
LDA, LDA![]() HDA, III
HDA, III![]() V, V
V, V![]() VI, VI
VI, VI![]() VII and VI
VII and VI![]() VIII
VIII
o At 5 TPa. [Back]
p The figures in brackets are the relative number of such rings. For the crystalline ices, they are from [2021].
q Data corrected to 0 °C, for direct comparison to ice Ih. The densities were determined at ≈ 80 K (ice Ih 0.932 g ˣ cm-3, ice Ic 0.943 g ˣ cm-3, LDA 0.937 g ˣ cm-3) [2032]. [Back]
r This ice has not been experimentally confirmed [Back]
Home | Site Index | Phase diagram | Crystal data | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2008 and last updated by Martin Chaplin on 27 March, 2020