
Amorphous water
Solid water can exist in several metastable non-crystalline amorphous forms, which have specific physical characteristics.
![]() Ultra-viscous water and the glass transition temperature
Ultra-viscous water and the glass transition temperature
![]() Low-density amorphous ice (LDA)
Low-density amorphous ice (LDA)
![]() High-density amorphous ice (HDA)
High-density amorphous ice (HDA)
![]() Very-high-density amorphous ice (VHDA)
Very-high-density amorphous ice (VHDA)
![]() The glassy state
The glassy state
'When the (water) vapour was condensed on a surface maintained at a temperature below -110 °C the condensate was vitreous'.
E. F. Burton and W. F. Oliver, 1935
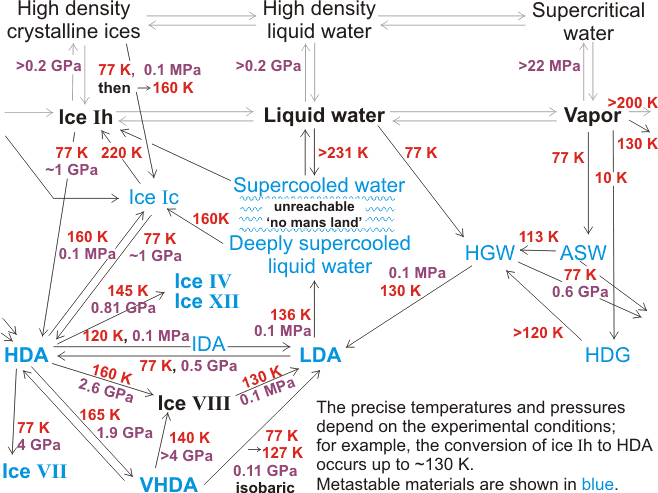
Amorphous ices are metastable forms of ice. They are kinetically stable in the pressure-temperature stability space of the thermodynamically stable crystalline ices. They can, therefore, exist for short or long periods of time (e.g. diamonds are metastable with respect to graphite at normal temperatures and pressures). As glasses are out-of-equilibrium systems, their properties depend on their preparatory history and are not defined by the state variables (the number of molecules, the system volume, and the temperature) [3726]. Due to the thermodynamic instablility of the amorphous ices, it is possible to avoid their formation [3606]; slow compression of hexagonal ice at 100 K giving proton-ordered ice IX , then proton-ordered and interpenetrating ice XV, and finally ice VIII. If cooled very rapidly, liquid water forms a glass e rather than crystallizing to hexagonal ice, for example, hyperquenched glassy water (HGW, [312e]). HGW is formed by the rapid spraying of a thin jet of μm-sized water droplets into freezing liquefied gas (for example, liquid propane), or onto a very cold solid substrate, about or below 80 K or by cooling capillary tubes containing bulk liquid water (≈ 100 μm diameter) with liquid helium at 4.2 K [1005]. These methods all involve cooling rates of greater than 105 K ˣ s-1. The glasses have structural, thermodynamic and density similarity with liquid water at 0 °C, due to their methods of formation and amorphous properties. A similar material is amorphous solid water (ASW), formed from the slow deposition of water vapor, at < 2 nm ˣ s-1, onto a very cold metal crystal surface below 120 K [900]. ASW (also called low-density glass, 0.94 g ˣ cm-3 solid) may contain considerable voids (up to 230 m2 ˣ g-1 [2425]) and dangling hydrogen bonds, which are removed by annealing under vacuum at 120-140 K when the glass converts to material indistinguishable from HGW or low-density amorphous ice (LDA, 0.94 g ˣ cm-3) at slightly higher temperatures. High-density glassy water (HDG, 1.1 g ˣ cm-3), formed by vapor deposition at 10 K [692] and subject to cosmic ray irradiation, d may be the most prevalent form of water in the universe. Notably, this 10-K ice has a higher density, than if if the vapor is deposited at 77 K, due to a significantly increased numbers of water molecules held at van der Waals distances (≈ 3.3 Å).
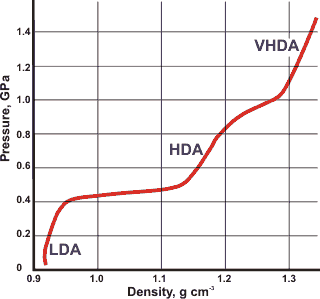
There is much interest in high-density ice (HDA, 1.17 g cm-3 at 0.1 MPa) and very-high-density ice (VHDA, 1.26 g ˣ cm-3 at 0.1 MPa) formed from LDA or crystalline ices. These last three amorphous ices (LDA, HDA, and VHDA) occupy three distinct megabasins in the energy landscape [1719] but are not required to obey the 'ice rules' and may contain a significant number of dangling bonds. Their structures may depend on the preparative method and thermodynamic history with well-relaxed (annealed) samples showing higher thermal stability [1953]. When annealed, they are expected to consist of fully hydrogen-bonded tetrahedral networks. This expectation is supported by their first-order phase transformation [2968]. The HDA to LDA transformation is a macroscopic phase separation rather than a homogeneous mixing of structures on the molecular scale. It is governed by the restructuring of the oxygen network rather than proton re-organization [2968], but highly dependent on the preparation and any prior relaxation of the LDA [2992]. Although some confusing data has been published, HDA and LDA are two distinct polyamorphs with VHDA being less distinct as it may relax toward HDA [3239]. HDA reaches the metastable equilibrium with LDA at 140 K and 0.1 GPa [3239].
Amorphous ices have been reviewed [1122, 1543, 1719] and their vibrational [1202] and diffraction scattering data compared [1546, 3501]. It was found by neutron scattering that ASW, LDA, and HGW were structurally very similar [1546], particularly if annealed. The amorphous ices have similar (unexpected) long-range order (but not short-range or intermediate-range order) and are not merely unstructured as typically frozen liquids [3227]. The HDA ![]() LDA phase transition has been examined [1635, 1682].
LDA phase transition has been examined [1635, 1682].
Solid water can thus exist in several non-crystalline forms (glasses), which have specific physical characteristics such as density and vibrational spectra. Although often treated as though they are homogeneous, there is no support for this assumption with many natural glasses being heterogeneous on the nano- or micro- scale with two or more separate phases [993]. As the amorphous materials, they mostly behave as glasses, which act as liquid depending on the time scale of observation. Many, but not all of the transformations, are shown below (mostly from [569]). All the amorphous solids are recovered to ambient pressure (0.1 MPa) at 77 K (liquid nitrogen) where they are (meta)stable for extended periods. While different preparations of the same density may have similar properties, it is likely that materials prepared by very different routes may differ. Some may simply be mechanically collapsed ice whereas others may have structures related to liquid water [1682], with transformations between these forms on annealing being possible but not obligatory. Using D2O below ∼210 K, but under confined conditions under pressure preventing freezing, two regions are found; a “low-density liquid” phase and a “high-density liquid” phase 16% more dense [2181]. There is no evidence that any of these amorphous ices are microcrystalline although sometimes they have been proposed as such. There are comprehensive reviews of the amorphous phases of ice and their transitions [569, 2033], a noteworthy simulation study of this polymorphism [590] and a critical review of the experimental data; particularly the diffraction data [1544]. Similarities to other tetrahedrally arranged substances (e.g., silicon and germanium) have also been noted [2392].
Relationships and transformations between the amorphous ices
[Back to Top ![]() ]
]
Water's metastable phase diagram
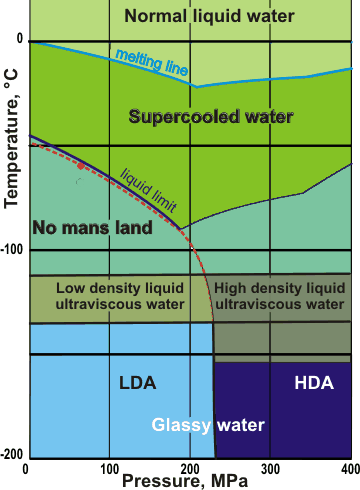
We base the metastable phase diagram above on the available
data plus related D2O work. It is slightly different from that in [3111, 3202] The 'phases' may be stable for hours or days. Metastable 'phase lines' may move with the direction and time taken for the processes. The (dashed) extended line is where there are maximum fluctuations (the 'Widom' line) and follows close to the upper bound of 'No man's land [3134]. It goes through the (disputed) position for the second critical point of water (see the red dot ) [2119, 2647]. Liquid water also changes its structure at about 200 MPa, but we have neglected the possible interference by VHDA . There is a non-equilibrium 'triple point' where LDA, HDA, and liquid water meet [2130], approximately as shown.
There are excellent review of possible glass-to-liquid transitions of the amorphous ices [2413, 3753]. A highly viscous deeply supercooled liquid water phase can be formed from either solid LDA, HDA, and VHDA indicating an extension of 'normal' supercooled liquid water with structural similarities. The transformation occurs on warming amorphous LDA, or compact amorphous solid water (ASW) deposited at 77 K [2593], to about 136 K [74, 137] or heating solid HDA at 1 GPa from approximately 130 K [1770]. On further slow heating above 136 K, the liquid material from compact ASW crystallizes at 144 K [2593]. On approach to the glass transition temperature (≈ 136 K, see below), there is a highly significant slow down in water's molecular dynamics. Additionally, there is a coupling of the thermodynamic and dynamical behavior [3182].
The transformation of HDA is reversible with the low-temperature high-density liquid water phase vitrifying on subsequent cooling [1770]. The use of X-ray photon-correlation spectroscopy (XPCS) in the small-angle X-ray scattering (SAXS) geometry has indicated that a first-order liquid-liquid transition, between low-density liquid and high-density liquid, occurs within the ultra-viscous regime [2930]. X-ray diffraction has shown that the transformations of the ultra-viscous liquid phases of LDA and HDA occur at about 140 K and 0.2 GPa [1762]. Ultra-viscous liquid HDA can exist down to 116 K under ambient pressure [2048, 3598]. A single sample of annealed HDA at 80 K can be warmed through 116 K to produce a high-density ultra-viscous liquid that can be allowed to convert to a low-density ultra-viscous liquid at 136 K and then to LDA if brought down below 136 K. The two distinct ultra-viscous states of water differ by about 25% in density [2048]. If ice-VIII is subjected to a rapid decompression (∼48 GPa/s) between 140 K and 165 K, it transforms to a low-density liquid that possesses a fully developed tetrahedrally coordinated network similar to low-density amorphous ices [3248].
This ultra-viscous deeply supercooled water has a fully developed local tetrahedrally coordinated network [3248], with a consistency variously described as "soft toffee" [312c] or "molten sherbet" [868]. It has a viscosity of fifteen orders of magnitude higher than that of the ambient liquid [1840] but still has a million-fold greater self-diffusion (at the still very low values of 2.2 ˣ 10-19 m2 ˣ s-1 at 150 K [334] and ≈ 1 ˣ 10-16 m2 ˣ s-1 at 160 K [1840]) than crystalline ice. The hundred-fold higher viscosity than that expected from its diffusivity may indicate the presence of long-lived crystallites within the deeply supercooled liquid [868]. It is not easy to investigate the relationships between these supercooled waters as there is an unobtainable 'no man's land' in the physical conditions where no liquid or glassy phase can be found and little experimental data exists [1903, 2236]. f ESR, however, indicates that a small fraction of the water molecules may be 'free', coexisting with cubic ice between about 160-230 K [1005]. The very existence of deeply supercooled liquid water, between about 136-160 K, is evidence that the glass transition temperature is the lower of those proposed (136 K, [312]), although characterized by only a small change in specific heat due to the high degree of strong hydrogen-bonding in the liquid. The glass transition may be linked with the unfreezing of the reorientation dynamics similarly to the disordered ices I, IV, V, VI, and XII [2601]. Deeply supercooled low-density liquid water is one of the strongest liquids known with heat capacity steps of < 1 J ˣ K-1. Deeply supercooled high-density liquid water gives a different glass transition temperature of 116 K [2048].
Deeply supercooled liquid water forms when amorphous ices are 'annealed' at temperatures just above 136 K. This lower density deeply supercooled liquid water is an excellent solvent for inert gas (e.g., xenon) atoms but is reported to be a very poor solvent for salt (LiCl) [1120] (however see below for the different behavior of the high density deeply supercooled liquid water). In addition, it has an excess entropy (1-2 kJ ˣ mol-1 ˣ K-1) much smaller than the entropy difference between liquid water and crystalline ice at 0 °C (22 mol-1 ˣ K-1) and an enthalpy of crystallization at 150 K (∼1.35 kJ ˣ mol-1) also much smaller than the heat of crystallization of liquid water (∼6 kJ ˣ mol-1) both indicating a high degree of tetrahedral order via intermolecular hydrogen-bonding [1840]. These properties indicate ES-like qualities with a possible molecular explanation presented elsewhere. At higher temperatures (≈ 165 K) LiCl becomes very soluble, and at high concentrations forms a deeply supercooled solution, as its interactions with ultra-viscous water overcome the strong water-water interactions present at lower temperatures [1225].
Further support for the presence of low-density ultraviscous liquid (LDL) and high-density ultraviscous liquid (HDL) has come from the study of dilute LiCl solutions in high-density ultraviscous liquid [3202]. Both liquids give sharp transitions to glass in dilute LiCl aqueous solutions above 0.01-mole fraction, in contrast to pure water. Deeply undercooled LiCl:6D2O water solution has been investigated as a function of pressure by neutron diffraction, small angle neutron scattering, and molecular dynamics simulations [3836]. The structure of the liquid evolves gradually between the s-HDA and s-VHDA boundary with no liquid-liquid transition.
Use of an ionic liquid hydrazinium trifluoroacetate (N2H5TFA) solution that interfered little in the apparent structure of water has achieved even more impressive supporting data [3571]. Using molecular dynamics, the contrast in structures between HDL and LDL is shown as NaCl partitions into the HDL and Ne partitions into the LDL fraction of supercooled water at temperatures below the second critical point [3623]; HDL thus showing a broken hydrogen-bonded structure and LDL showing an open clathrate-like structure. Several other materials possess a liquid-liquid transition such as phosphorus and silicon [2602]. There is further support from studies involving doping and isotope substitution [3532]. Using infrared spectroscopy and calorimetry, this solution is shown to undergo a sharp, reversible phase transition(≈ 190 K) between two homogeneous liquid states similar to those established for high- and low-density amorphous (HDA and LDA) water. Although this is not 'pure' water. it strongly supports the two-state hypothesis in pure water at higher temperatures.
[Back to Top ![]() ]
]
![Absorption spectrum of LDA ice, crystalline ice and liquid water, [2195] Absorption spectrum of LDA ice, crystalline ice and liquid water, [2195]](images/lda_oh_stretch.gif) LDA is now considered to include ASW, HGW, LDA-I, and LDA-II [1719] plus a more ordered form [2486]. LDA is prepared from glassy water, as above, by warming high-density amorphous ice (HDA) to just above 120 K at atmospheric pressure or by heating the high-pressure ice VIII phase at ambient pressure (Note however that some preparations of LDA differ from others [3318]. In particular, LDA-I is obtained by isobaric warming of HDA at ambient pressure and LDA-II is obtained by isothermal decompression of VHDA at 143 K. It is stable for months at 77 K and ambient pressure but transforms into cubic ice (probably stacking disordered ice) at about 150-160 K. The structure is unknown but consistent with LDA having an open architecture (primarily like ES) and structurally [718, 1284] and thermodynamically related to supercooled liquid water. It has a similar density to ES of 0.94 g cm-3 (LDA of D2O has a density of 1.04 g cm-3 at 0.1 MPa) and has tetrahedrally arranged hydrogen-bonded water molecules with four nearest-neighbors and clathrate cages (evidence presented
elsewhere). The dielectric relaxation time for LDA is 100-1000 seconds at 130 K, which is two orders of magnitude higher than that of VHDA, and indicates that the ice
rules are preventing more rapid orientational mobility [1447] similar to processes found in the crystalline ices. LDA may be considered highly coherent (somewhat ordered with collective oscillations) but not crystalline.
LDA is now considered to include ASW, HGW, LDA-I, and LDA-II [1719] plus a more ordered form [2486]. LDA is prepared from glassy water, as above, by warming high-density amorphous ice (HDA) to just above 120 K at atmospheric pressure or by heating the high-pressure ice VIII phase at ambient pressure (Note however that some preparations of LDA differ from others [3318]. In particular, LDA-I is obtained by isobaric warming of HDA at ambient pressure and LDA-II is obtained by isothermal decompression of VHDA at 143 K. It is stable for months at 77 K and ambient pressure but transforms into cubic ice (probably stacking disordered ice) at about 150-160 K. The structure is unknown but consistent with LDA having an open architecture (primarily like ES) and structurally [718, 1284] and thermodynamically related to supercooled liquid water. It has a similar density to ES of 0.94 g cm-3 (LDA of D2O has a density of 1.04 g cm-3 at 0.1 MPa) and has tetrahedrally arranged hydrogen-bonded water molecules with four nearest-neighbors and clathrate cages (evidence presented
elsewhere). The dielectric relaxation time for LDA is 100-1000 seconds at 130 K, which is two orders of magnitude higher than that of VHDA, and indicates that the ice
rules are preventing more rapid orientational mobility [1447] similar to processes found in the crystalline ices. LDA may be considered highly coherent (somewhat ordered with collective oscillations) but not crystalline.
![Arrhenius diagram of dielectric time constants, data from [2413] Arrhenius diagram of dielectric time constants, data from [2413]](images/ldahdatime.gif)
The oxygen pair correlation function is similar to that of supercooled liquid water [43], but the thermal conductivity behavior appears to differ [617] with LDA showing behavior more similar to a crystalline material rather than an amorphous glass [617, 1155]. The O-H stretch of 5% HDO in D2O (see above right) shows a broad absorption band due to the disorder in the sample. The peak is red-shifted in LDA from that of liquid water, indicating stronger hydrogen bonds [2195]. The red-shift indicates stronger hydrogen bonds. Although similarities exist, LDA is likely to have a somewhat different structure to ASW or HGW, which both show different glass transition behavior. Molecular dynamics (using the TIP4P/2005 model) of a small droplet (4.3 nm diameter, ≈ 1400 molecules) of 'no man's land water'/LDA gave many cyclic pentamers and hexamers as well as significant numbers of heptamers [3060].
Dielectric loss spectra (opposite, [2413]) give time constants that correspond to the rates of molecular reorientation. Surprisingly, LDA, HDA, and VHDA all give close to parallel lines in their Arrhenius plots with activation energies close to 34 kJ ˣ mol -1 [2048].
[Back to Top ![]() ]
]
HDA is considered to include the more thermally-stable expanded high-density amorphous ice (eHDA, made by decompressing VHDA, causing thermal relaxation, at 140 K) and unannealed high-density amorphous ice (uHDA) [1719]. uHDA is prepared by submitting low-pressure ices (Ih, Ic, XI a or LDA) to high pressure (˜ 1..5 GPa, ice Ih; ˜ 0.5 GPa, LDA) at low temperatures (for example at 80 K [2071] to 125 K [1122]). As with LDA, some preparations of HDA differ from others. Unannealed and expanded HDA ices have been compared using isotope substitution neutron diffraction [3730]. It is stable for months at 77 K and ambient pressure. HDA has a density of 1.17 g cm-3 at 0.1 MPa (1.31 g cm-3 at 1.0 GPa; HDA of D2O has a density of 1.30 g cm-3 at 0.1 MPa). The structural evolution on going from high-density amorphous ices to low-density state has been described [3577]. The uHDA structure is unknown and varies between preparations and probably consists of a mixture of constrained frozen materials, whereas the eHDA is glassy. The material appears to be heterogeneous on a length scale of nanometers [995]. It has been suggested that HDA forms due to formation of the kinetically arrested transformation, on pressurization, between hexagonal ice and high-density ice XV [3606]. However, the available data is not inconsistent with HDA (made from LDA) having the structure of crushed CS; the main feature appearing to be a puckering (collapsing) that increase the number of water molecules in the first coordination shell. b
Density (and volumes. mouse over) of liquid and crystalline water
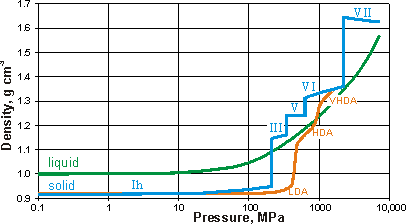
Effect of pressure on amorphous ices at 125 K, [1122]
The volume of glassy and crystalline water at about 100 K
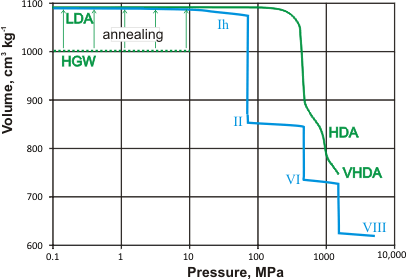
The collapsing
puckering of convex clathrate-type structures in the LDA ![]() HDA conversion involves a potential energy
barrier which may explain the difficulty of the reverse
HDA
HDA conversion involves a potential energy
barrier which may explain the difficulty of the reverse
HDA ![]() LDA conversion at low
temperatures (for example, 77 K) and sometimes put down as
a 'lynchpin' effect due to the presence of an extra non-hydrogen
bonded water molecule at about 0.345 nm [2895] (compare hydrogen-bonded HDA ≈ 0.269 nm and for LDA ≈ 0.274 nm) within the inner O···O coordinated shell. c The HDA
LDA conversion at low
temperatures (for example, 77 K) and sometimes put down as
a 'lynchpin' effect due to the presence of an extra non-hydrogen
bonded water molecule at about 0.345 nm [2895] (compare hydrogen-bonded HDA ≈ 0.269 nm and for LDA ≈ 0.274 nm) within the inner O···O coordinated shell. c The HDA ![]() VHDA conversion involves the presence of a second non-hydrogen
bonded water molecule with both at about 0.32 nm [2895] (compare hydrogen-bonded VHDA ≈ 0.272 nm) within the inner O···O coordinated shell.
VHDA conversion involves the presence of a second non-hydrogen
bonded water molecule with both at about 0.32 nm [2895] (compare hydrogen-bonded VHDA ≈ 0.272 nm) within the inner O···O coordinated shell.
Once a clathrate cage has puckered, there exists a more significant potential energy barrier to changes in puckering (for example, from a tetrahedral to an octahedral arrangement). Therefore as the initial puckering is expected to vary from site to site dependent on the strength (and weakness) of surrounding hydrogen-bonding, HDA formed this way is expected to be somewhat disordered. As HDA's structure appears to vary during annealing [394] (producing intermediate density amorphous ices, IDA [718] and in contrast to several early papers describing a first-order (meaning abrupt and direct) transition between LDA and HDA, see [569]), with preparation method [618b] (particularly with respect to time [618c]) and starting material [618d], data from diffraction and vibrational spectroscopy has to be interpreted carefully. A more stable annealed form of HDA, e-HDA (1.13 g ˣ cm3 0.1 MPa), has been prepared by annealing at about 0.2 GPa [1544]. This material appears to be homogeneous, completely converts to ice IX (the low-temperature form of ice III) on warming to ≈ 170 K at 100 MPa, possesses enhanced stability against transition to LDA at ambient pressure and is the best candidate for the solid form of high-density liquid water [2071]. e-HDA, on warming from below 100 K at 0.2 GPa forms ultra-viscous water at about 140 K before crystallizing into ice IX [2348]. A noteworthy property of HDA is that the thermal conductivity decreases from that of ice Ih, ice Ic or LDA during their pressurized densification. Usually, the thermal conductivity of materials increases with increasing pressure [618b].
LDA-nanodomains form with radii of ≈ 0.8 nm (similar in size to the (H2O)100 stranded clusters) in eHDA below ≈ 0.2 GPa. These nanodomains transform to crystalline ice IX nanodomains above ≈ 0.17 GPa. The LDA nucleation supports the first-order nature of the HDA → LDA transition and the liquid-liquid transition [3162].
Interestingly, ammonium fluoride (NH4 F), which has a similar hydrogen-bonded hexagonal crystal to hexagonal ice, converts, upon compression at 77 K, to a high-pressure phase isostructural with ice-four. Under the same circumstances, hexagonal ice converts to HDA rather than ice-four. Therefore, at least one form of HDA may be a ‘derailed’ state along the hexagonal ice to ice-four pathway [2861].
HDA is stabilized, relative to LDA, by the presence of the low molecular weigh polyol solutes, ethylene glycol, 1,3-propanediol, 1,2-propanediol, glycerol, xylitol, and D-sorbitol [3626]. This may be due to the destructive fit of the alkane OH groups to the tetrahedrally hydrogen bonded network of LDA.
[Back to Top ![]() ]
]
VHDA (first recognized in 2001 [693])
is prepared by submitting high-density amorphous ice (HDA)
at 77 K to isobaric heating to 160 K at 1.15 GPa [421].
On isobaric annealing of HDA between 0.3 and 1.9 GPa (with
temperature increasing from 77 K), VHDA appears to have formed
at 0.8 GPa with changes in density at higher pressures being
due to elastic compression [935]. The higher the annealing pressure and temperature of VHDA, the higher the thermal stability against crystallisation. Thus, this shows that the better-annealed material has fewer microcrystals within its structure [3253]. The LDA![]() e-HDA
e-HDA![]() VHDA transitions are reversible [1533]. Certainly, the phase transition from high-density amorphous ice (HDA) to very-high-density amorphous ice (VHDA) seems to be continuous rather than sudden (first order) [2665], at least at positive pressures [2895]. D2O behaves similarly to H2O, but with crystallization, to a mixture of high-pressure ices, taking place above 143 K rather than 140 K [1534]. Dielectric spectra indicate that VHDA can form a metastable ultra-viscous water state at 1 GPa and above 140 K [1160], but this liquid has unexpected properties compared with liquid water supercooled past the ice VI phase boundary and may be the high-density equivalent of the deeply supercooled low-density water described earlier. As VHDA is formed from HDA by structural relaxation (also known as rHDA),
it has been considered a more stable form of HDA [845], particularly above 0.8 GPa.[3240]) and appears to have a lower chemical potential than HDA for the entire range of pressures [2895].
It shows a greater extent of structuring than HDA and LDA
[675] and is
stable for months at 77 K and ambient pressure. It has a density
of 1.25 g ˣ cm-3 at 0.1 MPa (1.37 g ˣ cm-3 at 1.4 GPa), which may be indicative of the presence of some
ring penetration such as occurs in the similarly dense ice
six, although a simulation indicates otherwise [747]. c The dielectric relaxation time for VHDA is a couple of seconds at 130 K, which is two orders of magnitude less than that of LDA, and indicates that the proximity of non-hydrogen-bonded water molecules allows more rapid orientational mobility [1447].
VHDA transitions are reversible [1533]. Certainly, the phase transition from high-density amorphous ice (HDA) to very-high-density amorphous ice (VHDA) seems to be continuous rather than sudden (first order) [2665], at least at positive pressures [2895]. D2O behaves similarly to H2O, but with crystallization, to a mixture of high-pressure ices, taking place above 143 K rather than 140 K [1534]. Dielectric spectra indicate that VHDA can form a metastable ultra-viscous water state at 1 GPa and above 140 K [1160], but this liquid has unexpected properties compared with liquid water supercooled past the ice VI phase boundary and may be the high-density equivalent of the deeply supercooled low-density water described earlier. As VHDA is formed from HDA by structural relaxation (also known as rHDA),
it has been considered a more stable form of HDA [845], particularly above 0.8 GPa.[3240]) and appears to have a lower chemical potential than HDA for the entire range of pressures [2895].
It shows a greater extent of structuring than HDA and LDA
[675] and is
stable for months at 77 K and ambient pressure. It has a density
of 1.25 g ˣ cm-3 at 0.1 MPa (1.37 g ˣ cm-3 at 1.4 GPa), which may be indicative of the presence of some
ring penetration such as occurs in the similarly dense ice
six, although a simulation indicates otherwise [747]. c The dielectric relaxation time for VHDA is a couple of seconds at 130 K, which is two orders of magnitude less than that of LDA, and indicates that the proximity of non-hydrogen-bonded water molecules allows more rapid orientational mobility [1447].
Partial radial distribution functions
![The Intermolecular partial radial distribution functions of LDA and HDA and VHDA at 80 K, as derived from neutron diffraction, [421, 2303] The Intermolecular partial radial distribution functions of LDA and HDA and VHDA at 80 K, as derived from neutron diffraction, [421, 2303]](images/neutron-amorphous.gif)
Changes from HDA may occur by increased ordering, producing the more dense structure. There is a density-distance paradox with the nearest water-water distances increasing from 2.75 Å to 2.80 Å to 2.83 Å as the density increases for LDA, HDA and VHDA respectively. This increase is due to the number of non-hydrogen-bonded water molecules in the first hydration shell increasing from zero to one to two respectively [421, 1055]. These extra water molecules ease the network outwards in a manner seen in high-density liquid water. The intermolecular partial radial distribution functions of LDA, HDA, and VHDA at 80 K, as derived from neutron diffraction [421, 1546, 2303], are given on the right. The differences are due to an increase in interstitial molecules in the order LDA < HDA < VHDA. HGW and ASW behave very similarly to LDA The O-O radial distribution functions of LDA, HDA, and VHDA from X-ray data at 77 K has been determined recently, in agreement [3501]. The first coordination shell positions of the amorphous ices move further apart as the pressure increases and the amorphous ice densities increase [3501].
LDA |
HDA |
VHDA |
|
Formation Pressure |
0.1 MPa |
70 MPa |
1.1 GPa |
rO-O , Å |
2.750 |
2.780 |
2.803 |
Density, g ˣ cm-3 |
0.9435 |
1.1347 |
1.2616 |
The LDA-VHDA transformation has been studied using x-ray diffraction under isothermal compression and decompression. It was found to occur in two steps: a discontinuous transition between LDA and an HDA followed by a continuous change within this HDA involving structural reconfigurations and finally converging to VHDA [3774].
Interestingly, when warmed at different pressures between 0.3 and 2 GPa, VHDA recrystallizes into only the proton disordered ices III, IV, V, XII, VI, and VII in order of increasing pressure, but not into the proton ordered phases such as ice II [756] (in apparent contrast to HDA which can form ice IX [932]). Recent molecular dynamics simulation indicates that HDA may evolve continuously into VHDA and densification causes an increase in hydrogen-bonded rings containing 8-10 water molecules, due to more efficient packing [747], like ice-twelve with its 7- and 8- membered rings and similar density. If higher pressure is used (2 - 4 GPa) on HDA then high-pressure ices (ice-seven or ice-eight) are formed. VHDA shows structural similarities to high-pressure cold (>0.2 GPa, < 273 K; cooled and supercooled high-density liquid water) and hot water (to 6.5 GPa and 670 K [1001]) but possesses more extensive ordering. There is the possibility of a phase transition line between supercooled metastable liquid forms of LDA (called low-density liquid, LDL) and VHDA (called high-density liquid, HDL, and often referred to as the liquid phase of HDA before the discovery of VHDA). Such a situation is expected to end in a second (metastable) critical point [419, 432, 580, 2665]. The existence of this scenario is difficult to prove, and is disputed if becoming less so [2144 e,f, 3420], but is capable of (but not necessary for) explaining many of the low-temperature anomalies of liquid water. On the approach to the critical pressure and temperature, the ratio between thermal expansivity and specific heat (related to the Grüneisen parameter, Γ) diverges which is associated with the presence of a two-state system [3417].
A high-density two-dimensional form of amorphous water (sometimes referred to as a high-density two-dimensional ice) may be formed under ambient conditions on some surfaces, for example, BaF2 [2344].
[Back to Top ![]() ]
]
a The pressure transformation of ice XI (and ice VIII) has been modeled [749]. [Back]
b CS has a density of 1.00 and has 1/4 of its water dodecahedra puckered relative to ES (density 0.94 g cm-3). Full puckering of the remaining three water dodecahedra would give a density of 0.94 + 4 x 0.6 = 1.18 g cm-3, which is similar to the density of HDA of 1.17 g cm-3. If this puckering of the water dodecahedra is random with respect to the number of inner water molecules, this will produce a more disordered structure than LDA in line with the thermal conductivity data [617] and the recent finding of HDA's lack of a unique structure [618]. [Back]
c The activation energy for the
HDA ![]() LDA transition has been
estimated as equivalent to about one hydrogen bond [792a],
with that for the VHDA
LDA transition has been
estimated as equivalent to about one hydrogen bond [792a],
with that for the VHDA ![]() LDA
transition being equivalent to about two hydrogen bonds [792b]. The non-hydrogen-bonded water molecules may be due to separate interpenetrating hydrogen-bonded networks or a crushed hydrogen-bonded structure where nearby water molecules are linked by both hydrogen-bonding to third water molecules further away.
[Back]
LDA
transition being equivalent to about two hydrogen bonds [792b]. The non-hydrogen-bonded water molecules may be due to separate interpenetrating hydrogen-bonded networks or a crushed hydrogen-bonded structure where nearby water molecules are linked by both hydrogen-bonding to third water molecules further away.
[Back]
d A similar material may be prepared from low-density glass by lowering the temperature to 12 K when irradiated with 2-3 electron Å-2 at ambient pressure [957]. A glass of intermediate density (1.04 g ˣ cm-3) has been found using cooling rates of 110-271 K ˣ s-1 on diamond [972] and by modeling [498], and which may be related to the collapsed structure of water (CS) mentioned elsewhere. [Back]
Glass

e Glass. A glass is a nonequilibrium state of matter between that of a solid and a liquid. It is a non-crystalline amorphous solid that has a nanoscopically and microscopically disordered structure but many of the mechanical properties of a solid. Zanotto and Mauro have defined a glass as “Glass is a nonequilibrium, non-crystalline state of matter that appears solid on a short time scale but continuously relaxes towards the liquid state ”, with a more advanced definition given as "Glass is a nonequilibrium, non-crystalline condensed state of matter that exhibits a glass transition. The structure of glasses is similar to that of their parent supercooled liquids, and they spontaneously relax toward the supercooled liquid state. Their ultimate fate, in the limit of infinite time, is to crystallize" [3052a]. In the glassy state, the viscosity is extremely high (> 1012-1013 Pa ˣ s), conformational changes are severely inhibited and the material is metastably trapped in a solid but microscopically disordered state. A glass is rigid and brittle.
The glass transition temperature (Tg) is the temperature at which the molecular relaxation time reaches 100 s, and the viscosity of the system becomes 1012 Pa ˣ s. The relaxation time (τ, the return of the perturbed system back to equilibrium) of the material can distinguish between a glassy state (τ ≫1000 s) and a liquid (τ ≪1000 s); with glasses and liquids being isotropic and solids being anisotropic[3052b]. The flow rate of glassy water is about 10-14 m ˣ s-1 (≈ 30 µm in a century) compared with liquid water ≈ 4 m ˣ s-1. The glass transition is a time-temperature kinetic transition rather than a thermodynamic transition and different methods of cooling produce slightly different glass transition temperatures. Usually, there are discontinuities in physical, mechanical, electrical, thermal, and other properties of the material. Therefore, the value of Tg depends somewhat on the method of its determination; the glass transition (unlike true thermodynamic phase changes) occurring over a range of a few Kelvin. [Back]
f Liquid water may be studied in aqueous solution within 'no man's land' but only in tiny amounts, with a large surface area to volume as nanodrops, within emulsified aqueous solutions, or if confined in nanometer-sized pores [2257]. Under these circumstances, it may well not behave as 'bulk' water. [Back]
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV | XV | LSBU | Top
This page was established in 2004 and last updated by Martin Chaplin on 2 January, 2020