
Ice VIII substructure
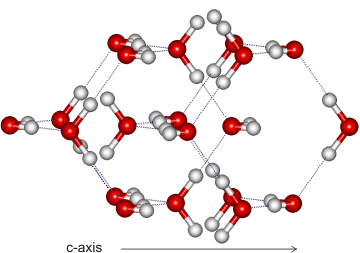
Ice-eight (ice VIII) forms from ice-seven (ice VII) on lowering its temperature (see Phase Diagram). The hydrogen-bonding is ordered and fixed as ice-seven undergoes a proton disorder-order transition to ice eight when cooled at about 5 °C; ice-seven and ice-eight having identical structures apart from the proton ordering. The proton ordering causes a slight distortion in the ice-seven cubic lattice (a,b slightly shorter, c slightly longer) resulting in a tetragonal crystal structure (I41/amd, 141; Laue class symmetry 4/mmm) where all of the water molecules are hydrogen-bonded to four others, two as a donor and two as an acceptor. Similarly to ice-seven, ice-eight consists of two interpenetrating cubic ice lattices. The molecular dipoles in these sub lattices point in opposite directions along the c axis making the crystal antiferroelectric. The bulk modulus and equation of state of ice VIII have been described [3300].
Ice VIII substructure variant
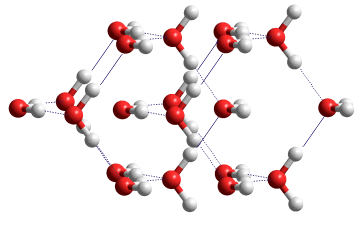
It is possible that a ferroelectric variant of this structure may exist (see left). In this crystal, the molecular dipoles in the sub-lattices point in the same direction along the c axis making the crystal ferroelectric (space group P42nm) [2358]. This structure is slightly less stable (≈ 1 kJ ˣ mol-1 ) than the antiferroelectric ice VIII but is likely to exist in domains at high pressure, particularly in an applied electric field [2358].
Ice eight
Ice VIII has a density of about 1.66 g cm-3 (at 8.2 GPa and 223 K [8]), which is less than twice the cubic ice density as the intra-network O····O distances are longer to allow for the interpenetration. Ice-eight's molar volume is slightly smaller (by 0.65 mm3 mol-1) than that of ice-seven along the phase transition line. Ice-eight has triple points with ice-six and ice-seven (5 °C, 2.1 GPa) and ice-seven and ice-ten (100 K, 62 GPa). The relative permittivity (dielectric constant) of ice-eight is about 4.
The crystal (shown on the right) has cell dimensions a, b = 4.4493 Å, c = 6.413 Å (90º, 90º, 90º; D2O, at 2.6 GPa and 22 °C [362]), containing eight water molecules per unit cell. All molecules experience identical molecular environments.
As the H-O-H angle does not vary much from that of the isolated molecule, the hydrogen bonds are not straight (although shown so in the figures).
Interactive Jmol structures are given.
Home | Site Index | Phase Diagram | Ices, introduction | Ice-Ih | Ice-Ic | Ice-Isd | II | III | IV | V | VI | VII | IX | X | XI | XII | XIII | XIV | XV | XVI| XVII | XVIII | Amorphous ice | LSBU | Top
This page was established in 2002 and last updated by Martin Chaplin on 20 September, 2018