
 Hydrogen
Bonding in Water (1)
Hydrogen
Bonding in Water (1)The hydrogen bond in water is a dynamic attraction between neighboring water molecules involving one hydrogen atom located between the two oxygen atoms.
![]() Water and Life
Water and Life
![]() Introduction
Introduction
![]() Water hydrogen bonds
Water hydrogen bonds
![]() Water hydrogen bond length
Water hydrogen bond length
![]() Water hydrogen bond direction
Water hydrogen bond direction
![]() Tetrahedrality
Tetrahedrality
![]() Hydrogen bond cooperativity
Hydrogen bond cooperativity
![]() Quantum effects
Quantum effects
![]() Water hydrogen bond 'wires'
Water hydrogen bond 'wires'
![]() Rearranging hydrogen bonds
Rearranging hydrogen bonds
![]() Bifurcated hydrogen bonds
Bifurcated hydrogen bonds
![]() Information transfer
Information transfer
![]() Hydrogen bonds and solubility
Hydrogen bonds and solubility
'Every hydrogen atom is thus linked to two oxygen atoms; undoubtedly it is linked more strongly to one of the oxygen atoms than to the other'
W. H. Zachariasen 1935 c
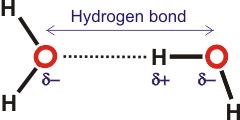
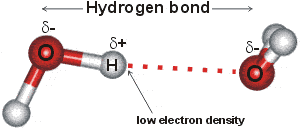
Water, with proteins and nucleic acids, is amongst the most important hydrogen-bonded substances. Hydrogen-bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a neighboring water molecule; generally, a proton shared by two lone electron pairs. In a water molecule (H2O), the oxygen nucleus with +8 charges attracts electrons better than the hydrogen nucleus with its +1 charge. Hence, the oxygen atom is partially negatively charged, and the hydrogen atom is partially positively charged. The hydrogen bond O-H···O may involve a wide range of bonding energies (4 - 120 kJ ˣ mol-1) and O···O contacts (0.238 to 0.300 nm). The hydrogen atoms are not only covalently attached to their oxygen atoms but also attracted towards other nearby oxygen atoms. This attraction is the basis of the 'hydrogen' bonds. Hydrogen bonding causes the collective ground state of liquid water to have an energy lower than the ground state found in single gaseous molecules.
 The water hydrogen bond is a weak bond, never stronger than
about a twentieth of the strength of the O-H covalent bond.
It is strong enough, however, to be maintained during thermal
fluctuations at, and below, ambient temperatures. a As the hydrogen bond in water is neither too weak nor too strong, it is sometimes regarded as having “Goldilocks” strength. The importance of this intermediate strength hydrogen bond is examined in depth within the 'Water and Life' page.
The water hydrogen bond is a weak bond, never stronger than
about a twentieth of the strength of the O-H covalent bond.
It is strong enough, however, to be maintained during thermal
fluctuations at, and below, ambient temperatures. a As the hydrogen bond in water is neither too weak nor too strong, it is sometimes regarded as having “Goldilocks” strength. The importance of this intermediate strength hydrogen bond is examined in depth within the 'Water and Life' page.
The lifetime of a single hydrogen bond is very short (≈ 1 ps, dependent on its definition). This is due to the large amplitude librations of the light hydrogen atoms that take them away from where the hydrogen bond attraction is high. Such broken hydrogen bonds will often simply reform. In liquid water, water molecules are connected within an extended dynamical hydrogen-bonded network with the individual hydrogen bonds vary between being shorter, straighter and stronger and longer, bent and weaker. They may even be broken for very short periods of time (< 100 fs).
The attraction of the O-H bonding electrons towards the oxygen atom leaves a deficiency on the far side of the hydrogen atom relative to the oxygen atom. The result is that the attractive force between the O-H hydrogen and the O-atom of a nearby water molecule is strongest when the three atoms are in close to a straight line and when the O-atoms are closer than 0.3 nm. In bulk water at any instant, it is expected that strong tetrahedrally-placed hydrogen bonds form a network (mesh) stretching throughout the liquid which delivers the water's physical properties, plus a smaller amount of isolated pockets of weakly or broken hydrogen-bonded water molecules [2695].
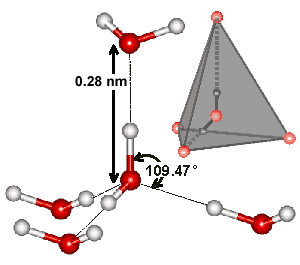
Each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds utilizing the hydrogen atoms attached to neighboring water molecules. These four hydrogen bonds optimally arrange themselves tetrahedrally around each water molecule as found in ordinary ice (see right). In liquid water, thermal energy bends and stretches and sometimes breaks these hydrogen bonds. However, the 'average' structure of a water molecule is similar to this tetrahedral arrangement, and endows water with its high cohesiveness. The diagram shows such a typical 'average' cluster of five water molecules. In the ices this tetrahedral clustering is extensive, producing crystalline forms. In liquid water, the tetrahedral clustering is only locally found and reduces with increasing temperature. However, hydrogen-bonded chains still connect liquid water molecules separated by large distances.
There is a balance between the strength of the hydrogen bonds
and the linearity that strong hydrogen bonds impose on the
local structure. The stronger the bonds, the more ordered
and static is the resultant structure. The energetic cost
of the disorder is proportional to the temperature, being
smaller at lower temperatures. This is why the structure of
liquid water is more ordered at low temperatures. This increase
in orderliness in water as the temperature is lowered is far
greater than in other liquids, due to the strength and preferred
direction of the hydrogen bonds, and is the primary reason
for water's rather unusual properties. [Back to Top ![]() ]
]
In liquid water, all water molecules have at least one hydrogen bond to neighboring water molecules with effectively no free water molecules under ambient conditions (i.e., 'free' molecules with no hydrogen bonds). Also, no isolated molecules exist more than 0.32 nm from any other molecule. There are two main hypotheses concerning the hydrogen-bonding of liquid water that divide water science. Either (a) water forms an effectively continuous three-dimensional network with the hydrogen bonds more or less distorted from their ideal three-dimensional structures, or (b) water consists primarily of a mixture of clusters of water molecules with different degrees of hydrogen-bonding in an equilibrium. Many properties of water are more easily explained using the latter model which is also supported by several experimental methods.
Water's hydrogen bonds
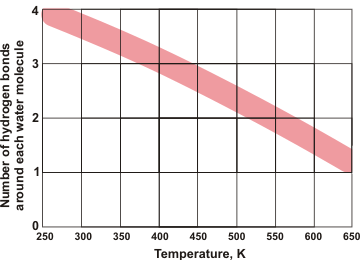
Water molecules in solid and low-temperature liquid water are exceptional, amongst hydrogen-bonding molecules, in having approximately twice as many hydrogen bonds as covalent bonds around each molecule and averaging as many hydrogen bonds as covalent bonds. Shown left is the number of hydrogen bonds around each water molecule as the temperature rises with the line-width showing the approximate disparity between different experimental methods (data from [2264]). Although there are reports of water surrounded by more than four hydrogen bonds (for example 5 or 6) these hydrogen bonds cannot be spatially accommodated around the central water molecule without being sited significantly further from the central oxygen (see below) plus with one or more of the original four hydrogen bonds being substantially weakened.
Five-coordinated hydrogen-bonded water
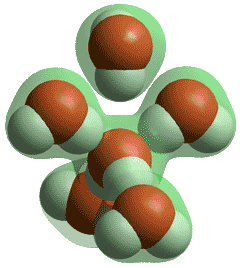
Thus, they can be bifurcated bonds where the bond is essentially shared between the water molecules (for example, two half -bonds rather than one full bond). No stable water cluster (for example within a crystal structure) has been found with the central water molecule 5-coordinated by hydrogen-bonding to five water molecules.
In water's hydrogen bonds, the hydrogen atom is covalently attached to the oxygen of a water molecule (492.2145 kJ ˣ mol-1 [350]) but has (optimally) an additional attraction (about 23.3 kJ mol-1 [168]. This is the energy (ΔH) required for breaking the bond and completely separating the atoms. It should equal about half the enthalpy of vaporization. On the same basis ΔS = 37 J deg-1 mol-1 [168]. (Lower enthalpies for the hydrogen bond have been reported [1369], varying between ≈ 6-23 kJ ˣ mol-1, with entropies ≈ 29-46 J deg-1 mol-1, depending on the assumptions made). Just breaking the hydrogen bond in liquid water, leaving the molecules essentially in the same position, requires only about 25% of this energy. This has been estimated at 6.3 kJ ˣ mol-1 [690], and only just over twice the average collision energy. a If the hydrogen bond energy is determined from the excess heat capacity of the liquid over that of steam (assuming that this excess heat capacity is attributable to the breaking of the bonds) ΔH = 9.80 kJ ˣ mol-1 [274]. Several estimates give the equivalent ΔG at about 2 kJ ˣ mol-1 at 25 °C [344]. However, from the equilibrium content of hydrogen bonds (1.7 mol ˣ mol-1 H2O), the equivalent ΔG is -5.7 kJ ˣ mol-1. The hydrogen bonding in ice Ih is about 3 kJ ˣ mol-1 stronger than liquid water (= 28 kJ ˣ mol-1 at 0 K, from lattice energy including non-bonded interactions) and evidenced by an about 4 pm longer, and hence weaker, O-H covalent bonds. However, the hydrogen bond strength in supercooled liquid water may be stronger than in ice [2020]. The hydrogen bond strength is almost five times the average thermal collision fluctuation at 25 °C)a to a neighboring oxygen atom of another water molecule and is far greater than any included van der Waals interaction. However, there are many van der Waals interactions tending to increase the density of water and together they influence the hydrogen-bonding network, that tends to decrease the density [3534].
Hydrogen bonds within heavy water (D2O) are stronger than for light water (H2O). Unexpectedly for such an important parameter, there is some dispute as to whether the hydrogen bonds in D2O and H2O are longer or shorter or the same length. One report states (opposite to earlier conclusions [554]) that D2O hydrogen bonds are longer (H····O 1.74 Å, D····O 1.81 Å at 23 °C [1485], but more linear; the weakening on lengthening being compensated by the strengthening on straightening) and D2O hydrogen bonds being more asymmetric (with the hydrogen atom more displaced away from the center of the O-H····O bond), more tetrahedral, more plentiful and stronger than in H2O [1485]. More recently the hydrogen bonds in D2O and H2O have been found to be about the same length due to compensatory quantum effects [1752]. Hydrogen bonds in T2O are expected to be stronger still. Thus given the choice, hydrogen bonds form with the preference
O-T····O > O-D····O > O-H····O
Water's hydrogen-bonding holds water molecules up to about 15% closer than if than if water was a simple liquid with just van der Waals dispersion interactions. However, as hydrogen-bonding is directional, it restricts the number of neighboring water molecules to about four rather than the larger number found in simple liquids (for example, xenon atoms have twelve nearest neighbors in the liquid state. Formation of hydrogen bonds between water molecules gives rise to large, but mostly compensating, energetic changes in enthalpy (becoming more negative) and entropy (becoming less positive). Both changes are particularly large, based by per-mass or per-volume basis, due to the small size of the water molecule. This enthalpy-entropy compensation [3476] c is almost complete, however, with the consequence that very small imposed enthalpic or entropic effects may exert a considerable influence on aqueous systems. It is possible that hydrogen bonds between para-H2O, possessing no ground state spin, are stronger and last longer than hydrogen bonds between ortho-H2O [1150].
The hydrogen bond in water is part (about 90%) electrostatic and part
(about 10%) electron sharing, that is covalent [96] (see discussion) and may be approximated by bonds made up of covalent HO-Hδ-····δ+OH2,
ionic HOδ--Hδ+····Oδ-H2,
and long-bonded covalent HO-··H––O+H2 parts with HO-Hδ-····δ+OH2 being very much more in evidence than HO-··H––O+H2,
where there would be expected to be much extra non-bonded
repulsion. The movement of electrons from the oxygen atom to the O-H antibonding orbital on a neighboring molecule (HO-Hδ-····δ+OH2) both weaken the covalent O-H bond (so lengthening it ) and reduces the HO-H····OH2 'hydrogen' bond. hydrogen-bonding affects all the molecular orbitals even including the inner O1s (1a1) orbital which is bound 318 kJ ˣ mol-1 (3.3 eV) less strongly in a tetrahedrally hydrogen-bonded bulk liquid phase compared to the gas phase [1227]. [Back to Top ![]() ]
]
Although the hydrogen atoms are often shown along lines connecting the oxygen atoms, this is now thought to be indicative of time-averaged position only and unlikely to be found to a significant extent even in ice.
Four-coordinated hydrogen-bonded water
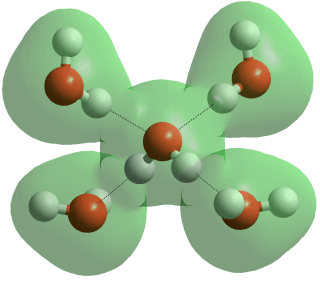
Liquid water consists of a mixture of short, straight [2405] and strong hydrogen bonds and long, weak and bent hydrogen bonds with many some way between these extremes. Short hydrogen bonds in water are strongly correlated with them being straighter [1083]. Proton magnetic shielding studies give the average parameters (given below right) for the instantaneous structure of liquid water at 4 °C; non-linearity, distances, and variance all increasing with temperature [458].
Note that the two water molecules below are not restricted to perpendicular planes, and only a small proportion of hydrogen bonds are likely to have this averaged structure.
Water dimensions
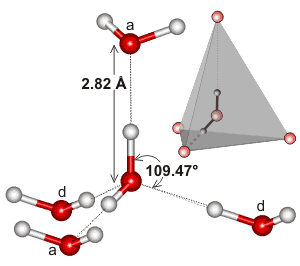
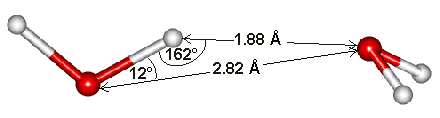
The hydrogen bond length of water varies with temperature and pressure. Although the (mean) H-O···O angle is indicated as 12°, it varies over a wide angle (considering both the quantum nature of the nuclei and thermal fluctuations) with ~50% probability between 4° and 24° at 250 K [3805]. The ~50% probability for the O···O distance is 2.7 Å to 3.1 Å. As the covalent bond lengths vary much less with temperature and pressure, most of the densification of ice Ih due to reduced temperature or increased pressure must be due to a reduction in the hydrogen bond length. This hydrogen bond length variation can be shown from the changes in the volume of ice Ih [818]. As hydrogen bond strength depends almost linearly on its length (shorter length giving stronger hydrogen-bonding), it also depends almost linearly (outside extreme values) on the temperature and pressure [818].
Note that in liquid water, the hydrogen-bonded arrangement of most molecules is not as symmetrical as shown here. In particular, the positioning of the water molecules donating hydrogen bonds to the accepting positions on a water molecule (that is, the water molecules behind in the diagram above, labeled 'd') are likely to be less tetrahedrally placed. b This is due to the lack of substantial tetrahedrally positioned 'lone pair' electrons, than those water molecules that are being donated to from that water molecule, (that is, the water molecules top and front in the diagram above, labeled 'a' [1224]. Also, the arrangement may well consist of one pair of more tetrahedrally arranged strong hydrogen bonds (one donor and one acceptor) with the remaining hydrogen bond pair (one donor and one acceptor) being either about 6 kJ ˣ mol-1 weaker [573], less tetrahedrally arranged [373, 396] or bifurcated [573]; perhaps mainly due to the anti-cooperativity effects mentioned elsewhere. Such a division of water into higher (4-linked) and lower (2-linked) hydrogen bond coordinated water has been shown by modeling [1349]. X-ray absorption spectroscopy confirms that, at room temperature, 80% of the molecules of liquid water have one (cooperatively strengthened) strong hydrogen-bonded O-H group and one non-, or only weakly, bonded O-H group at any instant (sub-femtosecond averaged and such as may occur in pentagonally hydrogen bonded clusters), the remaining 20% of the molecules being made up of four-hydrogen-bonded tetrahedrally coordinated clusters [613]. There is much debate as to whether such structuring represents the more time-averaged structure, which is understood by some to be basically tetrahedral [1024].
The interpretation of the structure of water in terms of strands and rings of doubly-linked hydrogen-bonded molecules [613] was not confirmed by a Compton scattering study [1083] where the data was consistent with 3.9 hydrogen bonds (Roo≤3.2Å) around each water molecule. Also, it has been disputed by another X-ray absorption spectroscopic study [690a], which presents a case for the 'non-, or only weakly, bonded O-H groups' to form the majority of O-H groups present and that these groups are more strongly bonded. Bowron challenges the above interpretation (that is, [613]) in the Discussion included in [746] and a Raman study supports the fully tetrahedrally-hydrogen-bonded model [875]. This dispute was thought to have been resolved by an ab initio molecular dynamics study [832] that shows 170 fs fluctuations of 2.2-fold strength between the two donor hydrogen bonds from each water molecule while the overall geometric connectivity is retained, in line with the hypothesis first presented above. However, this study [832] has attracted serious criticism [1159], leaving its conclusions seemingly unproven. Recent ab initio calculations of the x-ray cross section of liquid water show only 20% of broken hydrogen bonds are present [1059], other ab initio calculations show primarily tetrahedral coordinated water molecules [1654]. A novel force field for water, developed from first principles, gives 3.8 shared tetrahedrally coordinated hydrogen bonds per water molecule [1189]. Also, an ab initio quantum mechanical/molecular mechanics molecular dynamics simulation study shows that although the time-averaged hydrogen bonding is about four shared hydrogen bonds per water molecule, the instantaneous value is significantly lower at about 2.8 shared hydrogen bonds per water molecule [922]. Tetrahedrally-coordinated water seems most accepted at the present time [2095], but it is clear that a mixture of a minority of higher (4-linked) and a majority of lower (2-linked) hydrogen bond coordinated water can be fitted equally well with the experimental data [1350]. Even if the instantaneous hydrogen-bonded arrangement is tetrahedral, distortions to the electron density distribution may cause the hydrogen bonds to have different strengths [1979, 2095].
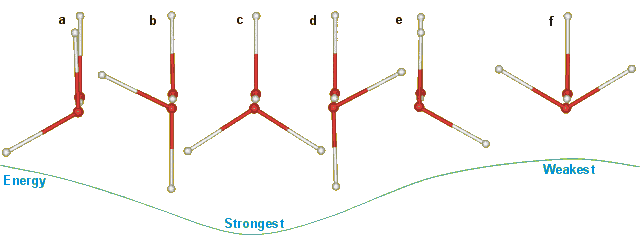
The latest molecular parameters for water are given elsewhere. The O····O distance in ice Ih varies between 2.75 Å (0 K) and 2.764 Å (253 K). The energy of a linear hydrogen bond depends on the orientation of the water molecules relative to the hydrogen bond. In an unstrained tetrahedral network (such as ice Ih) only the six structures below can arise with no structures at intermediate angles. The hydrogen bond energy depends particularly on the angle of rotation around the hydrogen bond, as below, due to the interaction between the molecular dipoles. Note that the hydrogen bonds in the structure pairs (a) and (e), and (b) and (d) have identical energies. Structure c has the minimal energy. In ice Ih with no net dipole moment, the configurations with extreme cis/trans ratios have 56.3% cis (i.e., a+e+f) or 64.7% trans (that is, b+c+d) but the calculated difference in energies was only 0.12% (0.06 kJ ˣ mol-1) [858]; much lower than the expected (several kJ ˣ mol-1) difference in energy between the limiting trans and cis structures c and f. As a, c and e involve protons in hydrogen bonds parallel to the c-axis, their increased strength relative to b, d and f may be causative to the (0.3%) shortened c-axis in the ice Ih unit cell.
Linked variation in bond lengths,
mouse over for ice VII [3168]
![variation of covalent and hydrogen bond length with oxygen-oxygen distance, from [1928] variation of covalent and hydrogen bond length with oxygen-oxygen distance, from [1928]](images/hydrogen_bond_length.gif)
There is a trade-off between the covalent and hydrogen bond strengths; the stronger is the H····O hydrogen bond, the weaker the O-H covalent bond, and the shorter the O····O distance [1928] (see right, where the bond lengths are equal if under at least 62 GPa pressure and ambient pressure (≈ 0.1 MPa) water has an average nearest oxygen distance of about 2.82 Å). Interestingly, this means that the O-H covalent part of the hydrogen bonds gets shorter as the temperature of the water increases. The weakening of the O-H covalent bond gives rise to a good indicator of hydrogen-bonding energy; the fractional increase in its length determined by the increasing strength of the hydrogen-bonding [217]; for example, when the pressure is substantially increased (≈ GPa) the remaining hydrogen bonds (H····O) are forced shorter [655] causing the O-H covalent bonds to be elongated. Hydrogen bond strength can be affected by electromagnetic and magnetic effects. The dissociation of water is a rare event, occurring only twice a day that is, only once in every 1016 times the hydrogen bond breaks.
The anomalous properties of liquid water may be explained primarily on the basis of its hydrogen-bonding [1530]. [Back to Top ![]() ]
]
An important feature of the hydrogen bond is that it possesses
direction; by convention, this direction is that of the shorter
O-H (![]() ) covalent bond
(the O-H hydrogen atom being donated to the O-atom acceptor
atom on another H2O molecule). In 1H-NMR studies, the chemical shift of the proton involved in the
hydrogen bond moves about 0.01 ppm ˣ K-1 upfield
to lower frequency (plus about 5.5 ppm further upfield to
vapor at 100 °C). It becomes more shielded with reducing strength of hydrogen-bonding [222, 1935]
as the temperature is raised. A similar effect may be seen
in water's 17O NMR, moving about 0.05 ppm ˣ K-1 upfield plus 36-38 ppm further upfield to vapor at 100 °C. Unfortunately, this is difficult
to use as a tool, however, due to the averaging of the shift
and the complexity of the system. The spin-lattice relaxation
times (T1, ≈ 3.6 s, 25 °C) of the water
protons is also a function of the hydrogen-bonding, being
shorter for stronger bonding. The effect of solutes, however,
shows the chemical shift and spin-lattice relaxation time
are not correlated, as solutes may reduce the extent of hydrogen
bonding at the same time as increasing its strength [281]. The spin-lattice relaxation time has been found to be two or three times greater than the spin-spin relaxation time, suggesting the presence of supramolecular structuring in the water [1664].
) covalent bond
(the O-H hydrogen atom being donated to the O-atom acceptor
atom on another H2O molecule). In 1H-NMR studies, the chemical shift of the proton involved in the
hydrogen bond moves about 0.01 ppm ˣ K-1 upfield
to lower frequency (plus about 5.5 ppm further upfield to
vapor at 100 °C). It becomes more shielded with reducing strength of hydrogen-bonding [222, 1935]
as the temperature is raised. A similar effect may be seen
in water's 17O NMR, moving about 0.05 ppm ˣ K-1 upfield plus 36-38 ppm further upfield to vapor at 100 °C. Unfortunately, this is difficult
to use as a tool, however, due to the averaging of the shift
and the complexity of the system. The spin-lattice relaxation
times (T1, ≈ 3.6 s, 25 °C) of the water
protons is also a function of the hydrogen-bonding, being
shorter for stronger bonding. The effect of solutes, however,
shows the chemical shift and spin-lattice relaxation time
are not correlated, as solutes may reduce the extent of hydrogen
bonding at the same time as increasing its strength [281]. The spin-lattice relaxation time has been found to be two or three times greater than the spin-spin relaxation time, suggesting the presence of supramolecular structuring in the water [1664].
The increased extent of hydrogen-bonding within clusters results in a similar effect; that is, higher NMR chemical shifts with greater cooperativity [436], shorter hydrogen-bonded O-H····O distances [1616], smaller atomic volume of the hydrogen atom, greater positive charge on the hydrogen atoms and greater negative charge on the oxygen atoms. The bond strength depends on its length and angle, with the strongest hydrogen-bonding in water existing in the short linear proton-centered H5O2+ ion at about 120 kJ mol-1. However, small deviations from linearity in the bond angle (up to 20°) possibly have a relatively minor effect [100]. The dependency on bond length is very important and has been shown to exponentially decay with distance [101].
Whether a hydrogen bond is considered broken or just stretched or bent should be defined by its strength but, as the isolated bond strength may be difficult to determine, this often remains a matter of an arbitrary definition based on distances and angles. Several geometric, energetic and combined definitions of hydrogen-bonding in water have been tested using models [2997]. An arrangement with strained geometry is very unlikely to last long. It may, however, occur during the breakage, formation or partner-switching (that is, bifurcation) of a hydrogen bond or arise transiently, due to thermal effects or other molecular interactions, in a long-lived hydrogen bond. The lifetime of a hydrogen bond (if more than 10-13 s) presents another measure of hydrogen bond formation, but this also suffers from uncertainties in the definition of its geometry. Broken hydrogen bonds do not last long enough to present a free hydroxyl (O-H) infrared spectrum (<10-14 - 10-13 s) [1687]. Indeed, when bonds lengthen or bend in real water, there will be the opportunity for the formation of weaker bonds elsewhere, and it is almost impossible to lose all interactions with the neighbors. Many hydrogen-bonding definitions have involved theoretically unsupported sharp cutoffs separating hydrogen-bonded from non-bonded molecules. Often these involve considerable transient breakage, which should be treated as an artifact of the definition employed [2417].
Some researchers consider the hydrogen bond to be broken if the bond length is greater than 3.10 Å or the bond angle less than 146° [173]. Other workers use more generous parameters; for example, in [848], the hydrogen bond length must be less than 3.50 Å and the bond angle greater than 120°, whereas others suggest hydrogen-bonding based on nearest neighbors [1432] or several combinations of lengths with angles (e.g., nearest neighbor O-O distances above 3.3 Å at θ = 0º = straight and above 2.5 Å at θ = 45º [613] ). The importance of choosing a correct definition for the hydrogen bonds has been examined [1240]. The simple distance criterion of 2.50 Å for the H····O distance was found very useful and cheapest in computational terms whereas methods based on energy proved poor. Adding further criteria, such as the bond angles, proved of marginal use [1240]. Six different hydrogen bond definitions are described in [1555] where they all gave the same qualitative picture of the spectroscopy. Using simulations, it has been proposed that purely geometric and energetic definitions are inaccurate as they may overestimate the connectivity and lifetime of hydrogen bonds and cannot distinguish improper relative orientations [1335]. Such overestimates may, however, be balanced by underestimates due to the cut-off parameters. The difference between the O-H and H····O bond lengths has also been suggested as an indication of hydrogen-bonding, where a water's hydrogen bond gives a difference around 0.75 Å with fluctuations (with bond angles ≈ 155° - 180°). The hydrogen bond can be considered broken with O-H H····O bond length differences varying with the bond angle (180° 1.67 Å; 135° 1.53 Å; 90° 1.40 Å). More simply for hydrogen bonds of significant strength (covering about 99% of water hydrogen bonds), and where the O-H H····O bond length difference is less than 1.25 Å [2025]. Use of network science determined that energetic criteria rather than geometric criteria was best for determining hydrogen-bond breakage [2994]. Some of the methods for defining water's hydrogen bond have been compared and reviewed [2028]. Finally, it is important to recognize that any definition of the hydrogen bond in terms of energy or geometry, is an approximation and cannot give accurate results or separate the overlap between the first and second-shell distributions that invariably occurs in a liquid. Further, the definition determines the number of 'hydrogen bonds' to be found around each water molecule.
Ab initio calculations indicate that most of the bonding energy still remains, and more bent but shorter bonds may be relatively strong; for example, one of the hydrogen bonds in ice-four (143°). Similarly, O····H-O interaction energies below 10 kJ ˣ mol-1 have been taken as indicative of broken hydrogen bonds although they are almost 50% as strong as 'perfect' hydrogen bonds and there is no reason to presuppose that it is solely the hydrogen bond that has been affected with no contributions from other interactions. Also, the strength of bonding must depend on the orientation and positions of the other bonded and non-bonded atoms and 'lone pair' electrons [525]. The use of different definitions for hydrogen-bonding gives rise to very different results for the amount and lifetime of the hydrogen bonds and the structure of putative clustering and solute hydration.
Many of the problems involved in describing hydrogen bonds by their geometry are removed if they are characterized by their strength or their charge-transfer [3479]. It was found that the O-H stretch frequency is inversely related to the hydrogen bond strength (ΔED→A) or the hydrogen bond charge-transfer (ΔCTD→A). These values (ΔED→A, ΔCTD→A) are shown to be able to predict water's hydrogen bond rearrangement dynamics.
[Back to Top ![]() ]
]
a The average molecular linear translational energy is RT/2. The average collision energy is RT (2.479 kJ ˣ mol-1). 2% of collisions have energy greater than the energy required to break the bonds (9.80 kJ ˣ mol-1, [274]) as determined by excess heat capacity. [Back]
b Tetrahedrality. The tetrahedral angle is 180-cos-1(1/3)°; 109.47122° = 109° 28' 16.39". Tetrahedrality (q, the orientational order parameter) may be defined as
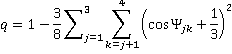
where ψjk is the angle formed by lines drawn between the oxygen atoms of the four nearest and hydrogen-bonded water molecules [169]; 1-2, 1-3, 1-4, 2-3, 2-4, 3-4. It equals unity for perfectly tetrahedral bonding (where all cos(φjk) = -1/3), 0.5 where two of the bonds are at tetrahedral angles, and the other two are random or broken, and averages zero (±0.5 SD) for random or broken arrangements, with a minimum ψjk value of -3. The density order parameter is described elsewhere, and these and other geometric order parameters characterizing the local structure of liquid water and its tetrahedral arrangement have been described and compared.
Similar equations may be used for testing trigonal-pyramidal order which is limited to the three nearest neighbors,
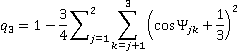
and trigonal bonding with ideal angles 120°
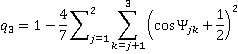
[Back]
c W. H. Zachariasen, The liquid "structure" of methyl alcohol, Journal of Chemical Physics,3 (1935) 158-161. [Back]
c Entropy-enthalpy compensation is caused by the key thermodynamic relationship
ΔG = ΔH - T ΔS
ΔG = (H2 - H1) - T (S2 - S1)
as when the bonding in the product(s) is stronger (H1> H2), it contains less disorder (S2 < S1) with the structure more firmly fixed. Therefore, the ΔH term goes down as the - T ΔS term goes up and consequentially the ΔG term moves little. [Back]
Home | Site Index | Hydrogen-bonding | More water hydrogen-bonding | Water and Life | Ices | LSBU | Top
This page was established in 2000 and last updated by Martin Chaplin on 18 December, 2019
The preferred Web Browser is Mozilla Firefox