
Earth: the Blue planet
Water covers about 71% of the Earth's surface, and this has allowed life to develop.
![]() Hard water, descaling, and desalination
Hard water, descaling, and desalination
![]() Water in the mantle
Water in the mantle
![]() Saltwater (seawater)
Saltwater (seawater)
![]() Water and global warming
Water and global warming
![]() Water cycle
Water cycle
![]() Water availability
Water availability
![]() Water use
Water use
Water, water, every where,
Nor any drop to drink.
Coleridge, 1798
A glacier-eroded U-shaped valley

Water and ice have been important in shaping the surface of the Earth due to erosion from rivers and glaciers (see left for the glacier-eroded U-shaped valleys in the Lake District of the UK). As well as adding to the beauty of the countryside, this increases the area of fertile areas for agriculture.
Water dissolves well in the mantle rocks. It plays a crucial role in the mantle by reducing friction between moving rock and reducing the melting point of such rock, lowering its density, and reducing its viscosity so encouraging the movement of the tectonic plates, seismic activity (earthquakes), and volcanic activity. For example, at 1000 MPa and 1200 °C, the solubility of H2O in the basaltic melt is 206 ± 9 g ˣ kg-1 [3097]. The presence of ice VII in diamonds points toward fluid-rich locations around the 660-kilometer upper boundary of the lower mantle (≈ 24 GPa) [3251].
Earth's tectonic plates
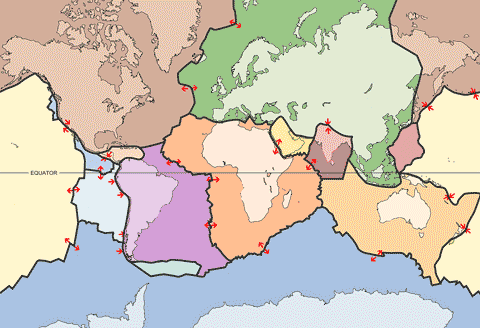
The outer shell of the Earth (lithosphere) consists of separate and distinct extensive rock layers (tectonic plates; see left), which float on the solid but fluid-like viscoelastic layer beneath (asthenosphere). The relative fluidity of the asthenosphere allows the tectonic plates to move (see red arrows left). The movement is caused by the upswelling of the asthenosphere, as particularly noticed in the mid-Atlantic ridge; a deep rift valley that spreads by about 2.5 cm per year. This motion is facilitated by water in and between the layers that reduce the friction. The water involved is drawn deep into the Earth (subduction) as one plate rides on top of another, taking many millennia to reappear in volcanic gasses.
Over time, water has reacted with the elements resulting in the minerals that make up the Earth's surface and the oceans' solutes [2270]. Most of the free water on Earth is saltwater (seawater, ~1.4 ˣ109 km3 ); an electrolyte solution that has a somewhat similar composition (if not total concentration) all over the planet. Its density [3769], thermophysical properties [2549] and light absorption [3435] have been described as has a comparison of sea water with pure water [3663]. Natural seawater salinity varies both in geographical position and in depth. It changes as a result of currents and mixing of water from different depths and densities, by rainfall and evaporation at the surface, by the freezing and melting of sea ice, by the freshwater discharged from rivers and glaciers, and indirectly due to local and non-local weather. Freshwater tends to float, and cold water tends to sink. The exact chemical composition of 'seawater' is unknown. The 'Absolute Salinity' of seawater is defined as the mass fraction of dissolved material. To avoid these small variations a 'standard' seawater has been defined, and its thermodynamic properties described [1452]. It is based on the major components of surface seawater taken from the North Atlantic.
Earth: Surface salinity and density; mouse over for
ocean currents and surface temperature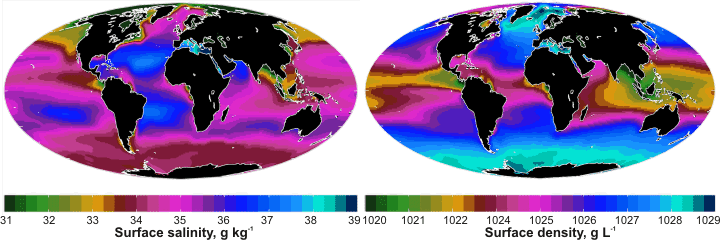
| There is a complex relationship between the salinity, density, temperature, buoyancy, currents, and physical geography. Surface salinity depends on the rainfall, ice formation, and evaporation rates and the density of the underlying water. The surface density is determined by the surface temperature and the surface salinity. Surface temperature (mouse over the diagram) is governed by the latitude and surface currents. The currents are driven by the rotation of the Earth, physical geography and seafloor topography, and differences in seawater buoyancy. |
The Absolute Salinity [2474] of this IAPSO 'Standard' seawater is 35.16504 g ˣ kg-1 exactly (1.16 molal ions; pH = 8.1; ionic strength 0.67 M; average molecular weight 31.4038 g ˣ mol-1, density 1.02346 g ˣ L-1; triple point 271.24 K, 521.56 Pa; melting point -1.91 °C; boiling point 100.56 °C; critical point 29.8 MPa, 680 K; osmotic pressure 2.576 MPa, surface tension 0.0728 N ˣ m-1 at 25 °C; but see also [3599] ); containing many ionic species, Na+ 10.78145 g ˣ kg-1, Mg2+ 1.28372 g ˣ kg-1, Ca2+ 0.41208 g ˣ kg-1, K+ 0.39910 g ˣ kg-1, Sr2+ 0.00795 g ˣ kg-1, Cl- 19.35271 g ˣ kg-1, SO42- 2.71235 g ˣ kg-1, HCO3- 0.10481 g ˣ kg-1, Br- 0.06728 g ˣ kg-1, CO32- 0.01434 g ˣ kg-1, B(OH)4- 0.00795 g ˣ kg-1, F - 0.00130 g ˣ kg-1, OH- 0.00014 g ˣ kg-1, B(OH)3 0.01944 g ˣ kg-1, and CO2 0.00042 g ˣ kg-1; all plus plus H2O 964.83496 g ˣ kg-1 [1452]. This is just over one molal salt ion and almost four times the concentration of salt in our blood, meaning that it cannot be used as the sole source of water for drinking, as we cannot get rid of that concentration of salt once ingested. Atmospheric gas is naturally dissolved in seawater, typically about 7, 13 and 0.3 ml STP liter-1 (20 °C) for O2, N2 and Ar respectively at 10 m depth and about 5, 14 and 0.4 ml STP liter-1 (20 °C) for O2, N2 and Ar respectively at 5000 m depth [2897]. The pKw of surface seawater is about 14.005 at 25 °C, and its redox potential is about +400 mV [3041]. Other saltwater may have greater osmotic pressure, such as the Great Salt Lake (≈ 24% salt, 37.5 MPa) and the Dead Sea (≈ 33% salt, 50.7 MPa).
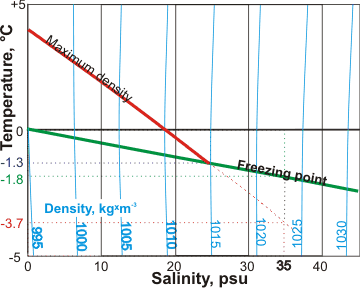
Seawater density and salinity
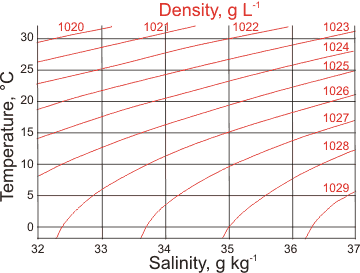
The surface of seawater begins to freeze at −1.91 °C with the ice excluding the salt. a This ice floats on the surface and the salt that is "frozen out" adds to the salinity of the remaining liquid seawater just below it, making it denser and causing it to sink towards the bottom, carrying dissolved oxygen with it. This, together with changes in salinity and temperature (both changing density, see left) has a major influence on ocean currents and behavior. For example, high-density water in the North Atlantic sinks, carrying oxygenated water to great depth, which then flows back towards the equator.
The temperature of maximum temperature decreases with increasing salt concentration (see below. psu are units on the Practical Salinity Scale b) until the water freezes at about -1.3 °C at a density about 1015 kg ˣ m-3.
The maximum density of saltwater
There has been recent concern over the acidification of the oceans and the consequential loss of dissolved carbonate from before the industrial revolution to the present (see below for 1751-1994) due to higher and rising atmospheric CO2 [2221]. The upper parts of the oceans are mostly supersaturated in calcium carbonate. This is important for the foraminifera protozoans as they possess CaCO3 coats that would otherwise dissolve. Only 9% of sea-water CO32- is free of complexation/ion-pairing. (see calcium carbonate equilibria).
Surface pH; mouse over for surface CO32-
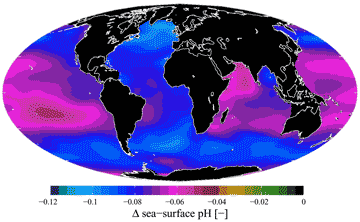
Ocean acidification, from [2222]
![Ocean acidification [2222] Ocean acidification [2222]](images/ocean_pH_2.gif)
If this continues then further carbon dioxide dissolution will alter the pH [2474] and carbonate chemistry of seawater and so reduce marine calcifying organisms.
CO2 + CaCO3 (solid) + H2O ![]() Ca2+(dissolved) + 2 HCO3-
Ca2+(dissolved) + 2 HCO3-
The relationship between atmospheric CO2, global warming and ocean acidification [2222] (see right) is very complicated with several feedback and feed-forward controls and different modeling approaches giving rise to different potential outcomes. 7000 years ago, the CO2 level was half what it is today. The possible error in the global warming response to any CO2 rise is of the same order as the CO2 rise itself [3167]. We hope that we may yet be able to avoid global warming exceeding 2 K. However, it is likely that continued increases in CO2 together with the related warming of the oceans will have substantial negative effects on marine ecosystems over the next 50 or so years [2352]. Currently, the main rise in atmospheric CO2 is down to China and developing countries with the EU's contribution dropping slightly. Deforestation, farming, and urbanization all increase the CO2 output. The lifetime of CO2 in the atmosphere is about ten years with about fifty times more in the oceans where it has a lifetime of hundreds of years.
The salt composition causes seawater to behave like pure water under extra pressure, and so many of the anomalies are reduced or lost [2179], particularly at higher salinity. For example, no maximum density is apparent as the seawater density increases as the temperature is lowered towards its freezing point.
Seawater does have some industrial uses such as in cooling power plants, and for conversion into drinking water.
[Back to Top ![]() ]
]
Projected global warming 1980-2050
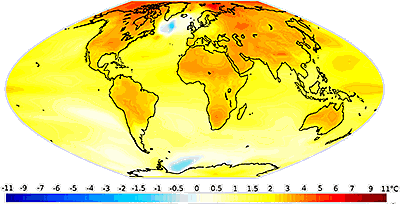
There has been much debate concerning global warming d over the last 50 years. Sometimes the science has been drowned out by the presence of many commentators chosen to 'balance' the arguments rather than their scientific expertise. Because of this, there is a danger of opinion rather than science unbalancing the debate. Modeling climate change is inexact, with many mostly understood, little understood and unknown variables. There are complex multi-factorial inter-relationships that cause the predictions to be imprecise with wide uncertainty.
The surface of the Earth warms mainly due to the Sun's shorter wavelength visible radiation (see spectrum). This radiation is absorbed, and some of the energy is radiated back outwards towards space as longer wavelength infrared radiation. The atmosphere is mostly transparent to visible radiation but contains several molecular gasses (such as H2O and CO2) that absorb infrared radiation. e
During Earth's existence, it has warmed and cooled many times due to natural events such as continental drift with
land occupation of the poles preventing warm water from the tropics melting and removing frozen water from these coldest regions of the world so allowing that to build up to cause glaciations [3692]. Some of these temperature shifts have been quite extreme, such as the Snowball Earth (650 Mya) when the Earth was almost entirely frozen, through many ice age events to the last ice age (from 2.6 Mya to about 0.024 Mya). These have involved mass extinctions and the evolution of new species. Ten of the warmest years on record (since 1880) have come in the last twenty years with five of the warmest in the last seven years (up to 2017). Cooling towards ice ages is slow as the ice builds up only slowly whereas warming can be much faster. The range over the last 800,000 years has been about 12 °C. The warming periods follow from increasing periods increasing CO2 giving a clear connection between these phenomena [3126].
Atmospheric CO2 varies in the seasons (high in Winter, low in Summer (due to photosynthesis), and between high in the northern hemisphere and low in the southern hemisphere (due to human activity and the size of the respective land masses). Recently, however, there has been concern over an apparent rapid warming over the last century caused by man's industrial activity in burning fossil fuels (see the recent rise in atmospheric CO2 above). The fossil fuels are known to be a cause due to their higher isotopic 12C content than would be found in other CO2 sources. Dire predictions of a dangerously warm Earth have been made for future decades, from NOAA (see above right). Other reports limit the warming to 1.5 °C from the mid-19th century to post 2015 warming, so long as present measures and pledges are honored [3061a]. It is important to note that global warming was preceded by increasing atmospheric CO2 at the end of the last Ice Age [3126]. The warming caused by this increase in CO2 has delayed the next ice age by about 100,000 years.
Recent global warming 1880-2014; mouse over for earlier years
![Global (land and ocean) surface temperatures, from [3061b]; mouse over for earlier data, from [3061d] Global (land and ocean) surface temperatures, from [3061b]; mouse over for earlier data, from [3061d]](images/gwarm.gif)
Additionally, reports of a recent “slowdown” in the increase of global surface temperature (see right for the last 135 yr, and mouse over for the last 1500 yr) have been challenged [3061b], leaving the field somewhat confused. The temperature increase is 0.85 °C since 1880, with about 20 cm rise in sea level and a 40% decline in sea ice.
A poorly understood component of global warming is the terrestrial carbon cycle [3061f]. This might accelerate slow climate change in the future due to human-driven land use changes in Earth's forests [3061c]. Aerosols, f produced with the warming CO2 and that have an overall cooling effect by reflecting incoming sunlight, have helped to reduce, by over a third, the warming effect from the CO2 emissions. As CO2 emissions are reduced, so will the cooling effect of air pollution [3254]. Trees are important in the control of CO2 and O2 in the atmosphere. For each ton of dry wood 1.851 ton of CO2 is removed from the atmosphere, being replaced by 1.393 ton of oxygen gas and 0.541 ton of water [3842], However, these amounts are reversed if the wood is burnt.
The maximum water in the air
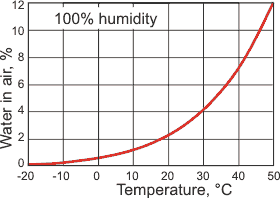
 Water is the most important absorber of the sunlight in the atmosphere (see elsewhere). In particular, it is a strong absorber of the infrared radiation from the Sun-warmed Earth before it radiates back into space. As warm air holds much more water than cold air (see left) an increase in the temperature of the atmosphere causes an increase in Earth's blanket of water vapor and a further warming effect. The 13 trillion tons of water in the atmosphere (≈ 0.33%
by weight; compare CO2 ≈ 0.04% by weight) is responsible for about 70% of all atmospheric
absorption of radiation. This is mainly in the infrared region where
water shows its strongest absorption. It contributes 50 % to 60 %
to the Greenhouse effect; more than twice that due to animal and anthropogenically associated carbon dioxide, ensuring a warm habitable planet,. Water operates a negative feedback effect, due to the cloud formation that reflects the sunlight away (see Quicktime movie) and the oceanic export of latent heat,
to attenuate global warming. Also, atmospheric water reduces the outgoing longwave radiation (OLR) from hotter surface temperatures such that the OLR is an essentially linear function of surface temperature over a wide range of temperatures, rather than obeying the Stefan-Boltzmann law (black-body radiation proportional to the fourth power of the surface temperature) [3441]. Rising atmospheric CO2 is accompanied by an increasing atmospheric humidity [3061e], due to (1) a positive feedback effect, (2) warmer air absorbing more water, and (c) man's activities. c Note that, without this Greenhouse effect, the average temperature on Earth would be well below the freezing point and the Earth would be in a permanent ice age. Although water vapor absorbs more radiated heat than CO2, the amount of H2O in the atmosphere is stabilized by its loss by increased rainfall. No such mechanism exists for CO2 which therefore may build up in the atmosphere and have a greater influence on global warming.
Water is the most important absorber of the sunlight in the atmosphere (see elsewhere). In particular, it is a strong absorber of the infrared radiation from the Sun-warmed Earth before it radiates back into space. As warm air holds much more water than cold air (see left) an increase in the temperature of the atmosphere causes an increase in Earth's blanket of water vapor and a further warming effect. The 13 trillion tons of water in the atmosphere (≈ 0.33%
by weight; compare CO2 ≈ 0.04% by weight) is responsible for about 70% of all atmospheric
absorption of radiation. This is mainly in the infrared region where
water shows its strongest absorption. It contributes 50 % to 60 %
to the Greenhouse effect; more than twice that due to animal and anthropogenically associated carbon dioxide, ensuring a warm habitable planet,. Water operates a negative feedback effect, due to the cloud formation that reflects the sunlight away (see Quicktime movie) and the oceanic export of latent heat,
to attenuate global warming. Also, atmospheric water reduces the outgoing longwave radiation (OLR) from hotter surface temperatures such that the OLR is an essentially linear function of surface temperature over a wide range of temperatures, rather than obeying the Stefan-Boltzmann law (black-body radiation proportional to the fourth power of the surface temperature) [3441]. Rising atmospheric CO2 is accompanied by an increasing atmospheric humidity [3061e], due to (1) a positive feedback effect, (2) warmer air absorbing more water, and (c) man's activities. c Note that, without this Greenhouse effect, the average temperature on Earth would be well below the freezing point and the Earth would be in a permanent ice age. Although water vapor absorbs more radiated heat than CO2, the amount of H2O in the atmosphere is stabilized by its loss by increased rainfall. No such mechanism exists for CO2 which therefore may build up in the atmosphere and have a greater influence on global warming.
Clouds reflect sunlight away from the Earth, trap the heat that radiates from the Earth's surface and re-emit absorbed heat, resulting in a complex and somewhat uncertain relationship between global temperature and cloud cover. The water content of the atmosphere varies about 100-fold between the hot and humid tropics and the cold and dry polar ice deserts (see above right and Quicktime movie). Clouds have a complex and controversial relationship in global warming as increased cloud cover insulates the planet so contributing to global warming by preventing heat loss, but also reflects back sunlight into space, so cooling the planet; with relative effects dependent upon the type and height of the clouds. If increasing water vapor leads to warmer temperatures, more water vapor will be incorporated into the atmosphere and warming, and water absorption will increase in a spiraling cycle. By contrast, if increased cloud cover leads to cooling, then the system will be stabilized. Changes in other gasses, such as CO2 will feed into these effects [2222]. On balance, it appears that increased atmospheric CO2 is feeding into a global warming spike in an otherwise cooling Earth.
Cloud cover and atmospheric vapor content, from NASA satellite observations
Cloud cover September 2014 Water vapor without clouds
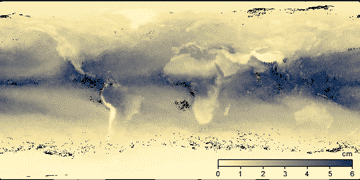
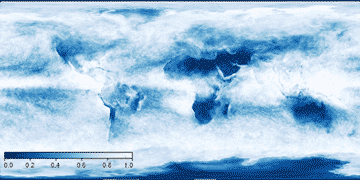
The current knowledge concerning ice formation within clouds has been outlined [2477]. Cloud condensation nuclei (aerosol particles, cloud seeds; such as sea salt, carbon black and 'dust') are particles with a dry diameter larger than 70 nm, which form cloud droplets at 0.22 % supersaturation. They are seen to have increased substantially during the industrial age [2424]. Over half of the atmosphere's cloud condensation nuclei are formed in situ from highly oxidized molecules, such as sulfuric acid, binding ammonia [2584].
Cloud condensation nuclei changes over the industrial age, from [2424]
![Cloud condensation nuclei changes over the industrial age, from [2424] Cloud condensation nuclei changes over the industrial age, from [2424]](images/cloud-droplet.gif)
Polar ice affects our climate as its white surface has a high albedo (reflectance) of up to about twelve times that of the open ocean. Any reductions of sea ice or the darkening of the ice caps, due to lack of fresh snow, exerts positive feedbacks in global warming.
The pattern of warming is very different at the North and South Poles (see above) with the Arctic losing ice, on average if not consistently, and the Antarctic gaining ice. The rate of warming in the Arctic is near twice the global average, but it is used by the media as though it is typical of global warming. It has been proposed that this difference between the poles is due to the ocean flow [3024]. Warmer water, caused by global warming, flows to the Arctic and causes the reduction in surface sea ice. However, the northward flow of this warmer water is balanced by the deep Northern Ocean's water returning south to well up in the Southern Ocean. Here, before being warmed at the surface, it is drawn northwards to be replaced by the deep, cold water. Thus, global warming causes the oceans to increase the warming of the Arctic whereas they reduce noticeable warming around Antarctica. It is no surprise that the Southern Ocean has shown little warming over recent decades, although warming may emerge in later decades as the deep Northern oceans are warmed. Note the patch of cold water in the North Atlantic (see above) due to buoyant salt-free water from the melting Greenland ice cap.
Sea level rise (mouse over for levels since the last ice age [3124])
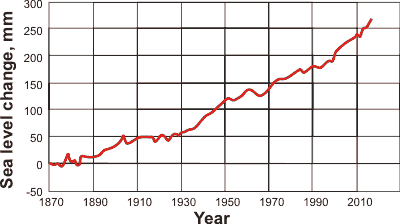
The mean sea level has risen ≈ 120 m since the last ice age (≈ 20,000 years ago, a melt of ≈ 5 ˣ107 km3 of land-based ice) and, without man's interference, would have likely continued to rise slowly (≈ 0.4 mm ˣ yr-1) towards that of the last interglacial period (+6 - +9 m, 130,000 to 115,000 years ago). Recently, the masses of both the Greenland ice sheet and the Antarctic ice sheet have rapidly and increasingly declined due to surface melting and iceberg calving. Between 2002 and 2016, Greenland and Antarctica shed approximately 305 km3 and 136 km3 of ice per year, respectively. This has caused the global sea level to rise by 0.8 mm ˣ yr-1 and 0.35 mm ˣ yr-1, respectively [NASA]. These contribute to the estimated current sea level rise of 3.4 mm ˣ yr-1 from satellite data [NASA]. Much of the difference is due to other melting land ice plus a smaller contribution from the expansion of water due to temperature rise. The high specific heat of water slows both the current global warming process and any future if now thought improbable, cooling recovery after greenhouse gas emissions are curtailed. It is expected that the oceans may rise by at least 28 centimeters by 2100. It should be noted that current land level rises and falls may be equal to the sea level changes in some places; for example, Scotland has a rising land mass of about 3 mm ˣ yr-1 due to rebound from the loss of ice sheets present during the last ice age.
The decline in the dissolved oxygen concentration in the oceans and coastal waters is part due to global warming and part due to other human activities modifying nutrients and aquatic species [3146].
A further point of dispute concerns the shape of the Earth. Higher gravity at the poles attracts the water from equatorial areas with lower gravity such that any rise in the oceans would not necessarily be evenly distributed [3212]. There would also be polar uplift due to the loss of land-based ice.
Massive iceberg on the horizon in the Southern Ocean

The rates of evaporation of both water droplets and ice crystals are limited by the diffusive transport of the vapor. Therefore, the evaporation from ice and snow (sublimation) is very similar to that of water drops [3446].. The rate of ice crystal sublimation is particularly important as it has a major impact on global climate, since surface ice and snow determine Earth’s albedo.
[Back to Top ![]() ]
]
The Water Cycle,
with the background from the Amazon rainforest
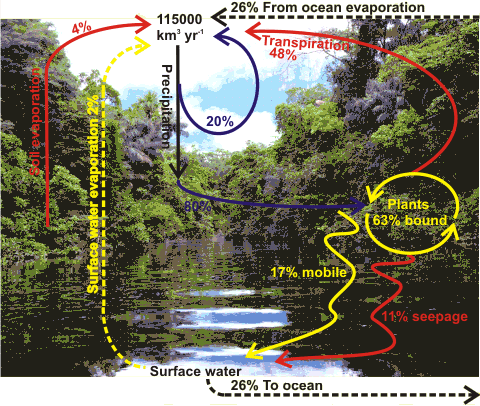
The high specific heat of water allows Earth's climate to be even-tempered, with an annual turnover of huge amounts of water in the water cycle (see right). An outline of the water cycle on Earth is thought to have been first recognized and observed by Leonardo da Vinci (1452-1519) [2494]. Now, it can be partially represented by the diagram right [2357]. Water has a lifetime in the atmosphere of about a week, on average, before precipitation. 20% of this precipitation over land evaporates before it reaches the ground. Of the remaining 80% about a fifth is run-off into the river system. Most of the remainder goes into the soil and vegetation, and 3/4 of this is returned to the atmosphere by transpiration with the remainder seeping to the river system or evaporating from the soil.
Transpiration is the major route for the return of water to the atmosphere over land with plants releasing about 300 molecules of water for every CO2 molecule captured during photosynthesis [2357].
Some seawater gets subducted, between tectonic plates, hundreds of kilometers down into the mantle taking with it small amounts of organic carbon and taking many millennia to reappear. Reactions occur under these extreme conditions (900 °C, 5.0 GPa), and materials like oil and diamonds may be formed [2453].
[Back to Top ![]() ]
]
Nordaustlandet ice cap

Only 2.5% of the water on Earth is fresh-water (< ≈ 0.5 g ˣ kg-1 salt in widely varying proportions; fresh water is not necessarily potable water). Most of that fresh water is frozen in the ice caps of Antarctica, Greenland, and Svalbard. Most of the remainder is inaccessible. Less than 0.9 % of the world's fresh water is in lakes, rivers, reservoirs and accessible underground sources (≈ 9.3 ˣ 104 km3) that can be used for drinking, cooking, hygiene, industry, and agriculture. Over half the worlds population has access to piped water on premises but about 10% still have to use surface water and other poor, potentially unsafe, sources. Although small as a percentage this 10% represents over 700,000,000 people. The map below shows the blue water (fresh surface water and groundwater) scarcity throughout the world [2603]. Addressing this is not cheap with achieving universal safely managed water and sanitation services by 2030 is estimated to require 114 billion US$ per year [3108].
Drought areas of the Earth, from [2603]
![The number of months per year in which blue water need was not met; 1996-2005 from [2603] The number of months per year in which blue water need was not met; 1996-2005 from [2603]](images/scarce.gif)
There have been great recent improvements in India and China. Even so, poor drinking water is the biggest threat to India’s public health, because 86% of India’s diseases are directly related to its quality [3353]. Much of the rural areas of Africa are poorly served and not improving year on year [2174]. Water scarcity is linked to famines and conflicts. Few people these days consider fresh water to be abundant, and generally they believe that there are likely to be shortages in the future. We need adequate, safe and affordable water to lead healthy lives, to industrialize, and to build secure livelihoods [2176]. It will be challenging to achieve this end for the poorest in society.
[Back to Top ![]() ]
]
Water is required for drinking (≈ 3 L ˣ day-1 ˣ person-1), cooking (≈ 10 L ˣ day-1 ˣ person-1), sanitation (≈ 20 L ˣ day-1 ˣ person-1), and washing (≈ 15 L ˣ day-1 ˣ person-1). In western cities, we are wasteful in our water usage, using 6-10 times these necessary amounts, whereas in rural third-world countries they get by on 10 L or less per day. The global domestic average is 184 L ˣ day-1 ˣ person-1 (455 km3 ˣ year-1), and the global industrial average is 300 L ˣ day-1 ˣ person-1 (739 km3 ˣ year-1 ) [2570]. Only about 1.7 % of the domestic water waste is reused, mainly for irrigation, with the rest being wasted through evapotranspiration or the sewerage system. Recent approaches to urban water management have been reviewed [2570]. The water we use domestically forms a very small amount of the fresh water required for modern life. As examples [2175], a liter of beer requires 300 liters (including growing the barley; a liter of milk requires 1000 liters (including farming the cows); a cup of tea requires 120 liters, a kg of potatoes requires 950 liters; a pair of leather shoes requires about 10,000 liters; a kg of beef requires up to 70,000 liters, and even a 2-gram microchip requires 32 liters. As water supplies become more expensive and subject to greater demand, ways are continually being sought to reduce these industrial water usage figures.
Water plays a central role in the world's religions as it is necessary for life and is a cleanser of body and spirit.
[Back to Top ![]() ]
]
a Sea ice consists of pure water ice plus air pockets and very salty liquid water inclusions that gradually drain away with time. It differs from glacial ice, which does not contain any saltwater inclusions. [Back]
b The Practical Salinity Scale. A full chemical analysis of seawater is too time-consuming for routine use. The Practical Salinity Scale 1978 has been developed so that routine salinity determination may be simply made for a comparison between different studies [2484]. The Practical Salinity Scale defines salinity in terms of the conductivity ratio of a sample to that of a standard solution of 32.4356 g of KCl at 15 °C in a 1 kg solution. A sample of seawater at 15 °C with a conductivity equal to this KCl solution has a salinity of exactly 35 practical salinity units (psu). Other conductivities are associated with salinities using empirical relationships, roughly equivalent to g ˣ kg-1 solution. [Back]
c Using oil or gas as a fuel produces approximately equal molar amounts of CO2 and H2O. [Back]
d Global temperatures are gathered and combined using a number of sources. Land temperatures are determined in the air above ground and not the ground itself; typically from within an automated weather stations. Sea surface measurements are from ships and static or drifting buoys. Weather satellite observations measure atmospheric temperatures using microwaves. The World Meteorological Organisation recommends defining the temperature of a location as the average of the maximum and minimum temperatures recorded during a 24 hour period. Historical data obtained by different methods are adjusted by various factors and use the baseline set by these modern observations. These adjustments reduce the trends but do not change the conclusion that global warming is real. [Back]
e The stretch vibration of O2 and N2 involves no change in dipole moment (always zero), so there is no infrared absorption. The asymmetric stretch of CO2 involves continually changing dipole moment such that it absorbs radiation of the same frequency as the dipole moment changes.
Asymmetric stretching causes dipole movement
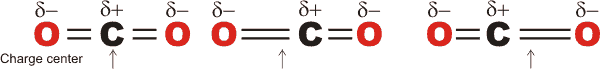
[Back]
f A. Voiland, Aerosols: Tiny particles, big impact, https://earthobservatory.nasa.gov/features/Aerosols, accessed 5 Sept 2019. [Back]
Home | Site Index | Water and life | Water and astrobiology| Hydration, water and health | LSBU | Top
This page was established in 2014 and last updated by Martin Chaplin on 6 January, 2020