
Pipe in need of descaling

Descaling is the removal of limescale for industrial and domestic water users in hard water areas.
![]() Calcium carbonate equilibria
Calcium carbonate equilibria
![]() Water softening
Water softening
![]() Magnetic descaling
Magnetic descaling
Hard water is water that has high calcium or magnesium ions content (>≈ 1.2 mM ) acquired during its passage through calcium or magnesium-containing rock such as limestone or chalk. The amount of dissolved calcium and magnesium in water determines its "hardness", expressed as the equivalent amount of calcium carbonate in parts per million (mg ˣ L-1). Classification into 'hard' or 'soft" water is not internationally standardized; water having < 60 mg ˣ L-1 ( < 60 ppm) calcium carbonate equivalent is generally regarded as 'soft whereas water having > 120 mg ˣ L-1 ( > 120 ppm) calcium carbonate equivalent is generally regarded as 'hard'. Rainwater is exceptionally soft (≈ 1 mg ˣ L-1 calcium carbonate equivalent) and seawater is extremely hard (> 6000 mg ˣ L-1 calcium carbonate equivalent). In domestic settings, hard water is often indicated by a lack of suds formation when soap is agitated in water, as the minerals precipitate the soap as scum. Hard drinking water is generally not harmful to health, seems protective against cardiovascular disease, and can usefully serve as a dietary supplement for calcium and magnesium. It can, however, cause the formation of limescale in both domestic and industrial boilers and water treatment. Most wines (> 198 mg ˣ L-1), beers (> 240 mg ˣ L-1) and bottled waters (e.g., Evian (313 mg ˣ L-1), Perrier (418 mg ˣ L-1), and San Pellegrino (605 mg ˣ L-1) use hard water sources [3459].
Limescale (consisting of mainly calcium carbonate, plus calcium sulfate, barium sulfate, calcium phosphate, magnesium hydroxide, zinc phosphate, iron hydroxides and silica, dependent on the geographical area) is a problem in heated water systems wherever 'hard' water is obtained from limestone or chalk countryside. It is formed primarily because the solubility of calcium carbonate decreases with increasing temperature. Limescale is only a problem if calcium carbonate deposits calcite crystals, which may form directly or subsequent to metastable hexagonal and fibrous vaterite crystal formation. Hard water may be treated, and limescale can be removed by water softening or the process of descaling.
[Back to Top ![]() ]
]
On heating a solution containing dissolved bicarbonate, the following reaction occurs irreversibly due to the loss of gaseous CO2.
Ca(HCO3)2 (aqueous) ![]() CaCO3 (solid)↓ + H2O + CO2 (gas)↑
CaCO3 (solid)↓ + H2O + CO2 (gas)↑
The thermodynamic formation/dissolution of solid CaCO3 from/in aqueous solution involves the four CO2 equilibria plus water dissociation plus four Ca2+(aq) relationships (given below with data at 25 °C) [2182].
| 2 H2O |
Kw = [H3O+] ˣ [OH-] |
Kw = 0.991 ˣ 10-14 mol2 ˣ L-2 |
| CO2 (g) + H2O |
|
KH = 29 [IAPWS] |
| CO2 (aq) + H2O |
|
KD = 590 |
| H2CO3 + H2O |
|
K1 = 0.25 mM |
|
Ka1 = 0.45 µM (apparent) | |
| HCO3- + H2O |
|
Ka2 = 0.047 nM |
| CaCO3 (solid) |
|
KS = 3.35 nM (calcite) KS = 4.49 nM (aragonite) |
| CaCO3 (aq, ion-pair) |
|
KD = 0.703 mM |
| CaHCO3+(aq, ion-pair) |
|
KD2 = 96.6 mM |
| CaOH+(aq, ion-pair) |
|
KD3 = 71.0 mM |
There is a total of nine equilibria in eleven unknowns (CaCO3 (aq), H3O+, Ca2+(aq), CaHCO3+ (aq), CaOH+ (aq), OH-, HCO3-, CO32-, CO2 (g), CO2 (aq), H2CO3). A further equation is the charge balance (positive charges = negative charges)
H3O+ + 2 Ca2+(aq) + CaHCO3+ (aq) + CaOH+ (aq) = OH- + HCO3- +2 CO32-
so allowing all of the equilibrium concentrations to be calculated, given one of them. However, it is not straightforward as there are three different KS values dependent on the crystal form, the equilibrium constants are disputed/inaccurate, and the concentration-dependent hydration equilibria involving bound water are not included.
The solubility of CaCO3 with temperature [2178]
![Solubility of CaCO3 with temperature [2178] Solubility of CaCO3 with temperature [2178]](images/caco3_crystals.gif)
[CaCO3](S) is given the value unity in the equation. Note that Ca(HCO3)2 is not thought to exist as solid or by ion association and evaporating stoichiometric solutions gives CaCO3 and CO2 rather than a solid Ca(HCO3)2. Slightly different values for the solubility product (KS) are provided in the literature. The crystal form of the CaCO3 also has an effect with aragonite being about 16% more soluble (KS 34% greater) and vaterite being twice as soluble (KS four times greater) [2178]. All these solubilities reduce with increasing temperature, which is why dissolved CaCO3 forms scale in heated pipes. Also, these solubilities increase with increasing dissolved CO2 and more acidic pH. KS is an equilibrium value and does not relate to the kinetics of any dissolution/ precipitation. As calcite is the least soluble, at equilibrium at ambient temperature where aragonite is about one kJ ˣ mol-1 less stable [107], aragonite and vaterite in water convert to calcite. Aragonite is, however, kinetically if not thermodynamically, favored to precipitate at higher temperatures.
Water saturated with CaCO3 in equilibrium with pCO2 = 0.0003 atm forms about 10 µM CO2 (aq) solution of pH 8.3, containing about 0.5 mM Ca2+, 5 µM CaCO3 (aq), 5 µM CaHCO3+(aq), 20 nM CaOH+(aq), 10 µM carbonic acid (H2CO3), 1.0 mM HCO3-, 10 µM CO32-. This is close to the reaction (CaCO3 + O2 + H2O ![]() Ca2+ + 2 HCO3-) of 0.5 mM CaCO3 dissolved to give 0.5 mM Ca2+ and 1 mM HCO3-.
Ca2+ + 2 HCO3-) of 0.5 mM CaCO3 dissolved to give 0.5 mM Ca2+ and 1 mM HCO3-.
A range of different CaCO3–based functional poly-crystalline materials are produced by natural processes involving soluble additives to amorphous CaCO3 [3613]. An example is nacre (mother of pearl), in pearls and shells formed by aragonite platelets.
[Back to Top ![]() ]
]
Water softening is the process of removing the Ca2+ and Mg2+ ions. The usual method for this is to use an ion exchange resin (for example, sulfonated polystyrene) to replace them by Na+ ions.
Na+··· Resin··· Na+ + Ca2+(aq) ![]() Resin:::Ca2+ + 2 Na+(aq)
Resin:::Ca2+ + 2 Na+(aq)
The resin is usually made of small beads. After it is full of Ca2+ and Mg2+ ions, it is regenerated by washing with an excess of NaCl salt.
Resin:::Ca2+ + 2 Na+(aq)) (excess) ![]() Na+··· Resin··· Na+ + Ca2+(aq)
Na+··· Resin··· Na+ + Ca2+(aq)
[Back to Top ![]() ]
]
It is widely reported that magnetic fields may halt or reverse scale build-up [2213, 2676]. The literature is somewhat confused with some reporting that orthorhombic aragonite crystals have a higher density but, although intrinsically harder, are less prone to form hard scale on surfaces [104], whereas other papers report aragonite is more troublesome forming the harder scale on surfaces [2183]. There seems to be little experimental literature to decide one way or another, and in any case it is the relative (kinetic) ability of the precipitating particles to stick to surfaces rather than themselves, under the prevailing physical (e.g., electrical and magnetic fields) and chemical (e.g., Mg2+ and Fe3+ content) conditions, that is of overriding importance.
CaCO3 crystal |
Density, g cm-3 |
Solubility product, M2 25 °C |
||
| Calcite | Trigonal (R3c) | 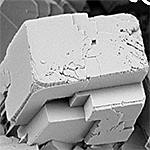 |
2.701 |
3.4 ˣ 10-9 |
| Aragonite | Orthorhombic (Pmcn) | 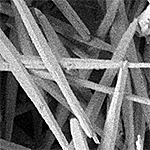 |
2.904 |
6.0 ˣ 10-9 |
| Vaterite | Hexagonal (P63/mmc) |  |
2.560 |
1.2 ˣ 10-8 |
The mechanism by which the magnetic field produces its effect seems down to the presence of disordered hydrated CaCO3 aggregates [1954], a forming liquid emulsions which may be affected by the magnetic field and so convert into different prenucleation clusters and hence different structures on crystallization [1955].
The solubilities of the different crystal forms of CaCO3 follow the expected trend in so far as the higher the number of crystal edges and corners, the greater the solubility. Once formed, crystals
are kinetically (if not thermodynamically) stable for hundreds
of hours. By drawing water through a static magnetic field
(B ≈ 0.1 T, ![]() B≈ 10 T ˣ m-1, it has
been shown that the initial amount of aragonite formed is
significantly increased over calcite in samples with and without
the presence of dissolved iron [107],
although this aragonite eventually changes to calcite [555].
A separate experiment has shown that drawing a pure solution of calcium carbonate and bicarbonate through static magnets (0.16 T) for 5-30 min increases the precipitate formed on degassing the excess dissolved carbon dioxide [1043]. Magnetic treatment has been shown to affect the crystal surfaces of both aragonite and calcite [1689] and increases the rate of aragonite crystallization over that of calcite [2184].
B≈ 10 T ˣ m-1, it has
been shown that the initial amount of aragonite formed is
significantly increased over calcite in samples with and without
the presence of dissolved iron [107],
although this aragonite eventually changes to calcite [555].
A separate experiment has shown that drawing a pure solution of calcium carbonate and bicarbonate through static magnets (0.16 T) for 5-30 min increases the precipitate formed on degassing the excess dissolved carbon dioxide [1043]. Magnetic treatment has been shown to affect the crystal surfaces of both aragonite and calcite [1689] and increases the rate of aragonite crystallization over that of calcite [2184].
The direction and variation of the magnetic fields have also been shown to be important [555], with crystal size decreasing with increasing magnetic field [623]. A different group has showed agreement in a recent study where under similar conditions (B = 0.5 T, flow rate = 0.1 m ˣ s-1) the magnetic field produces mainly a mixture of aragonite (44%) and vaterite (42%) whereas without it well-crystallized calcite (34%) is formed with little aragonite (14%) [252]. It has been proposed that the smaller water cluster size, being more reactive, hydrates the calcium and carbonate (particularly; see a recent supporting study [793]) ions more effectively and so encourages aragonite nucleation [110]. Alternatively, the magnetic field may cause a surface or orientation effects on the growing crystals [980]. It is possible, however, that the major effect is the magnetically induced competitive formation of hydrated silica in suitable solutions that then absorbs calcium ions [353]. The pipe material and its surface were also found to be important [1586].
There are many devices on the market for the magnetic treatment of water for the removal of such limescale. The sales success of these devices would seem to indicate that some work as promoted, at least under some circumstances.
Magnetic treatment of water is claimed to cause four effects: [104]
Many tests, mainly utilizing single pass systems, however, have proved negative [212]. Recirculatory systems, with prolonged magnetic exposure, give more supportive results. Rapid movement (1200 rpm) in a strong magnetic field (4.75 T) had a significant effect compared with the movement or field alone [105]. A smaller number of larger crystals causes the effect as nucleation is suppressed and crystal growth is enhanced. It is possible that the result is due to magnetically-enhanced corrosion promoting the release of Fe2+ that, even at ppm, reduces calcite but has no effect on aragonite production. We know that inhibiting the thermodynamic transformation of aragonite into calcite. Fe2+ may be used as a threshold inhibitor by industry. However, the amount of dissolution, required by this theory, has not been found. Magnetic treatment devices that create additional turbulence may enhance anti-scaling effect [109], perhaps by encouraging precipitation in the bulk (due to a combination of magnetically modifies hydration and the shifting of charged particles in the magnetic field [645]) rather than by deposition. Another contributing factor may be the lowering of the surface tension, multiple passes producing increased lowering up to about 8% [735]. A potential (= v ˣ B ˣ L where L is the distance between detecting electrodes) is generated when a dilute electrolyte flows (v) through a transverse magnetic field (B, greatest when the flow is orthogonal to the magnetic field). This may increase colloidal coagulation. A recent well-controlled study has shown that scaling can be reduced by a few percents by even one pass through a simple magnetic device but that it is difficult to increase this effect to more than about 20% even with extensive recirculation [259]. This study also showed an optimum in the flow rate as at too high a flow rate the magnetic field was encountered only briefly, an effect recently confirmed [555]. Recently, the presence of dissolved oxygen has been shown important for the production of the magnetic effect for forming aragonite rather than calcite [970], and for initiating scaling [1046]. It may be assumed that many of the studies described on this page did not control for oxygen content, so their effects may have been moderated by the varying dissolved oxygen contents. b
There are 'electronic' devices, related to the above purely magnetic devices, that use weak electromagnetic signals utilizing a coil wound around a pipe. A square-wave pattern is often used as it effectively contains many frequencies from a few Hz to 100 kHz [657]. This changes the magnetic field in a manner similar to a number of rapid passes past a very weak static magnet. However, the changing electric field will also contribute to the effect, as shown using a pulsed electric field [799]. Recently,13.56 MHz at 2 V has been found to work well [1626].
[Back to Top ![]() ]
]
a These sloppy objects are known as DOLLOPs (dynamically-ordered liquid-like oxyanion polymers) [1954]. [Back]
b Oxygen can be removed using sodium sulfite plus cobalt chloride (catalyst). [Back]
Home | Site Index | Magnetic and electromagnetic effects | LSBU | Top
This page was established in 2002 and last updated by Martin Chaplin on 4 June, 2019