
Some cyclic alkanes have been synthesized that correspond to the water clustering in the icosahedral water clusters.
Polycyclic alkanes
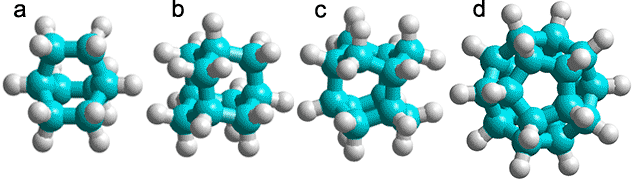
a) C8H14, bicyclo[2,2,2]octane; b) C10H16 adamantane (tricyclo[3.3.1.13,7]decane); c) C12H18, iceane (wurtzitane, tetracyclo[5.3.1.12,604,9] dodecane) [1843]; d) C20H20, dodecahedrane (fullerane-C20-Ih, undecacyclo[9.9.0.02,9.03,7.04,20.05,18.06,16.08,15.010,14.012,19.013,17] icosane) [1844].
C280H120 structure
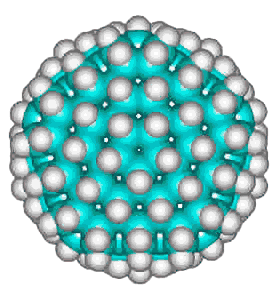
Dodecahedrane
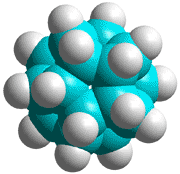
The basic expanded network structure of the icosahedral water cluster is mechanically strong, having close to tetrahedrally-positioned bonds, and the central dodecahedron as an alkane (see left) has been synthesized. The complete cluster could be found in the, as yet unsynthesized, alkane C280H120; made up of twenty C14 tetrahedral sub-structures. Such molecules are also known as 'nanodiamonds'. The stability and electronic spectra of the icosahedral carbon nanoparticles C20H20, C100H60 and C280H120 have been calculated [1845]. It was calculated that hydrogenation stabilized the structures that otherwise preferred an 'onion' like (Russian doll) layered carbon shell structure [1846]. The first discussion of similar icosahedral carbon clusters was in 1993 [1847].
Using the AMBER force-field, the average C-C and C-H bond lengths and bond angles for C280H120were 1.533 Å (SD 0.014 Å), 1.091 Å (SD 0.0001 Å) and 109.46° (SD 1.47°) respectively.
Interactive structures are given (Jmol).
C70H60 structure
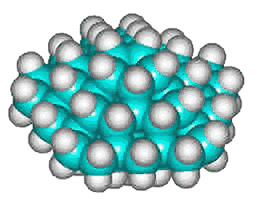
There could also be the quarter-structure C70H60 (Jmol), shown left made up of five C14 tetrahedral sub-structures (the dihedral angle at each edge of a tetrahedron is 70.529°, leaving this structure just 7.356° (2%) short of that required for perfect tetrahedra forming a circle). Using the AMBER force-field, the average C-C and C-H bond lengths and bond angles of C70H60 were 1.534 Å (SD 0.0026 Å), 1.091 Å (SD 0.0002 Å) and 109.47° (SD 0.68°) respectively.
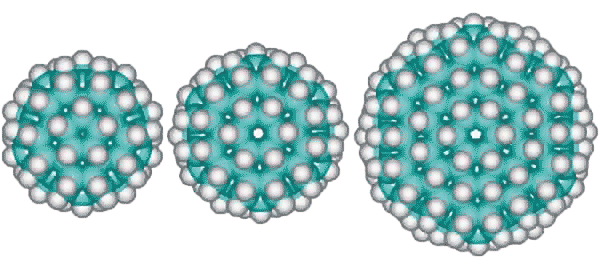
Other derivative fulleranes are also possible such as (from the left below) C100H60 (20 ˣ C5H3 units) based on the inner cluster with a central carbon dodecahedron, C120H72 (24 ˣ C5H3 units) based on the inner cluster with a central carbon tetrakaidecahedron (51262) and C336H144 (24 ˣ C14H6 units) based on the complete tetrakaidecahedron cluster.
C100H60, C120H72 and C336H144 alternative fulleranes
Home | Site Index | Fullerene solutions | LSBU | Top
This page was established in 2002 and last updated by Martin Chaplin on 19 March, 2020