
Comparison of H2S and H2O, calculated using the Restricted Hartree-Fock
wave function (RHF) using the 6-31G** basis set.
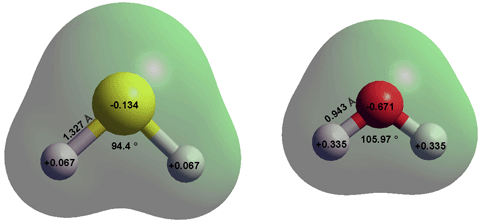
Hydrogen sulfide a (H2S) is a colorless, dense, flammable, reactive, and corrosive gas (boiling point, -60 °C; melting point, -85.5 °C, critical temperature 100 °C, critical pressure 8.97 MPa, critical density 347.6 kg ˣ m-3) with a characteristic unpleasant and highly toxic odor of rotten eggs even at low concentrations (>~0.5 ppb). Dangerously, at very high concentrations, its smell is undetectable. It is naturally produced and utilized in living metabolism, f in the putrefaction of organic matter, and from volcanic gases. It is almost non-polar with dipole moment 0.97 D in the gas phase and 1.25 D in the aqueous phase [3846]. In aqueous solution, it acts as a weak acid. Its structure (see top right) and properties are very different from H2O, in contrast to the apparent similarity, as an analog, at first glance. H2S is very soluble in common organic solvents.
H2S is only slightly soluble in water with a minimum solubility at about 200 °C (1 atm, mole fraction (H2S + SH- + S2-) = 0.0005; mole fraction at 0 °C and 25 °C being 0.00375 and 0.0019 respectively); the solubility behavior being similar to other non-polar gases. It behaves as a weak acid. g The (disputed c) high pKa2 means that the concentration of S2- in aqueous solution is generally negligible.
H2S + H2O ![]() SH- + H3O+ pKa1 = 6.98 (25 °C) b
SH- + H3O+ pKa1 = 6.98 (25 °C) b
SH- + H2O ![]() S2- + H3O+ pKa2 = ~17 (25 °C) c
S2- + H3O+ pKa2 = ~17 (25 °C) c
The pKa1 drops to a minimum of about 6.5 at about 100 °C. e H2S is a weak reducing agent. It forms colorless solutions that turn yellow with the formation of insoluble sulfur.
S0 + 2H+ + 2e- ![]() H2S E°' = +0.17 V d
H2S E°' = +0.17 V d
S0 + H2O + 2e- ![]() HS- + OH- E°' = -0.478 V [70]
HS- + OH- E°' = -0.478 V [70]
S0 + 2e- ![]() S2- E°' = -0.508 V [70]
S2- E°' = -0.508 V [70]
2 H2S + O2 ![]() 2 S0 + 2 H2O slow (pH <~ 6)
2 S0 + 2 H2O slow (pH <~ 6)
2 HS- + O2 + 2H+ ![]() 2 S0 + 2 H2O slow (pH >~ 6)
2 S0 + 2 H2O slow (pH >~ 6)
H2S + 2 O2 ![]() HSO4- + H+ very slow
HSO4- + H+ very slow
Comparison of H2S and H2O dimers, calculated using the Restricted
Hartree-Fock wave function (RHF) using the 6-31G** basis set.
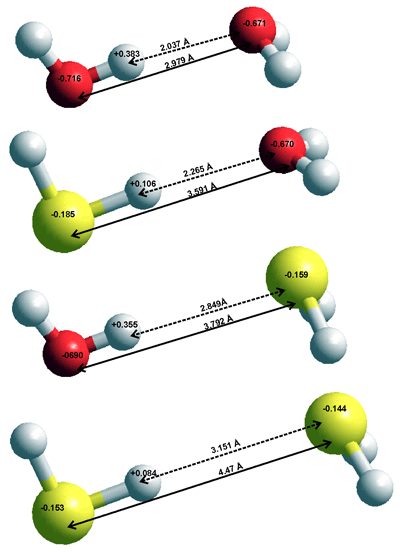
The low atomic charges (see top right), large molecular dimensions, and low polarity of H2S means that H2S - H2O interactions, including their very weak hydrogen bonds, are far smaller than H2O - H2O interactions. This is due to the increased dispersive interactions being more than compensated by the very decreased electrostatic interactions. The in vacuo dimers are given on the right with interactions:
H2O···H2O ≫ HSH···OH2 > HOH···SH2 ≫ H2S···H2S
In H2S solutions the water···water interactions are far stronger than the interactions involving H2S, so that the mixed clusters are negligible with H2S molecules generally excluded from the water hydrogen bonding network and with the H2S preferring a dodecahedral water clathrate environment [3846].
Even if single hydrogen bonds transiently form between H2S and H2O, the H2S molecule cannot sustain any further hydrogen bonds whereas the H2O molecules prefer having four hydrogen bonds.
Dissolved H2S acts as a powerful surfactant lying at the surface with the sulfur atom pointed away from the bulk water [3846].
[Back to Top ![]() ]
]
a The spelling 'Hydrogen sulphide' is not recommended [IUPAC] but still acceptable. [Back]
b F. J Millero, The thermodynamics and kinetics of the hydrogen sulfide system in natural waters, Marine Chemistry, 18 (1986) 121-147. [Back]
c W. Giggenbach, Optical spectra of highly alkaline sulfide solutions and the second dissociation constant of hydrogen sulfide. Inorganic Chemistry, 10 (1971) 1333-1338; this high value is disputed with others claiming a value around pKa2 = 14, R. S., Ramachandra and L. G. Hepler, Equilibrium constants and thermodynamics of ionization of aqueous hydrogen sulfide. Hydrometallurgy, 2 (1977) 293-299. [Back]
d O. Kabil and R. Banerjee, Redox biochemistry of hydrogen sulfide, Journal of Biological Chemistry, 285 (2010) 21903-21907. [Back]
e J. A. Barbero, K. G. Mccurdy and P. R. Tremaine, Apparent molal heat capacities and volumes of aqueous hydrogen sulfide and sodium hydrogen sulfide near 25°C: the temperature dependence of H,S ionization, Canadian Journal of Chemistry, 60 (1982) 1872-1880. [Back]
f E. Cuevasanta, M. N. Möller and B. Alvarez, Biological chemistry of hydrogen sulfide and persulfides, Archives of Biochemistry and Biophysics, 617 (2017) 9-25. [Back]
g The pKas of H2Se and H2Te are 3.7 and 2.7 respectively [3888b], with that for H2Te thirteen orders of magnitude greater than that for H2O. [Back]
Home | Site Index | Solubility of non-polar gases | Hydrophobic hydration | Protein hydration | Nucleic acid hydration | Aqueous biphasic systems | Gas-liquid interface and nanobubbles | LSBU | Top
This page was established in 2020 and last updated by Martin Chaplin on 4 March, 2020