
Alpha-cyclodextrin
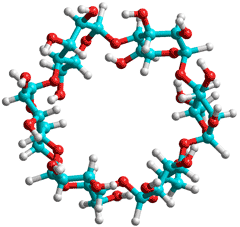
Cyclodextrins are crystalline non-reducing cyclic glucose oligosaccharides.
'La sustance desséchée est très avide d'eau'
'the dried material is very hungry for water' M. A. Villiers, 1891 a
Cyclodextrins result from the cyclomaltodextrin glucanotransferase (E.C. 2.4.1.19; CGTase) catalyzed degradation of starch. They form soluble inclusion compounds with less-hydrophilic molecules that fit into their cavities. Their structures [918] and use in the food industry [1576] have been reviewed. There are three common cyclodextrins with 6, 7 or 8 D-glucopyranosyl residues (α-, β-, and γ-cyclodextrin respectively) linked in a ring by α-1,4 glycosidic bonds. The glucose residues have the 4C1 (chair) conformation. All three cyclodextrins have similar structures (that is, bond lengths and orientations) apart from the structural necessities of accommodating a different number of glucose residues. They present a bottomless bowl-shaped (truncated cone) molecule stiffened by hydrogen-bonding between the 3-OH and 2-OH groups around the outer rim. The hydrogen bond strengths are α-cyclodextrin < β-cyclodextrin < γ-cyclodextrin.
Alpha-cyclodextrin
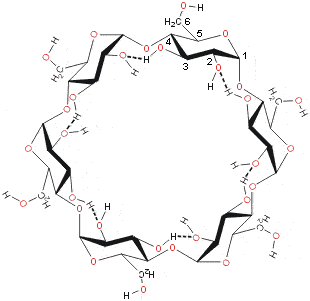
The flexible 6-OH hydroxyl groups are also capable of forming linking hydrogen bonds around the bottom rim, but these are destabilized by dipolar effects, easily dissociated in aqueous solution and not typically found in cyclodextrin crystals. The hydrogen bonding is all 3-OH (donor) and 2-OH (acceptor) in α-cyclodextrin but flips between this and all 3-OH (acceptor) and 2-OH (donor) in β- and γ-cyclodextrins [918].
Cyclodextrin shape
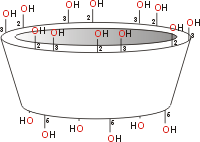
The cavities have different diameters dependent on the number of glucose units (empty diameters between anomeric oxygen atoms given in the diagram below). The side rim depth (shown below in the diagrams) is the same for all three (at about 0.8 nm).
Comparison of alpha-, beta- and gamma-cyclodextrin
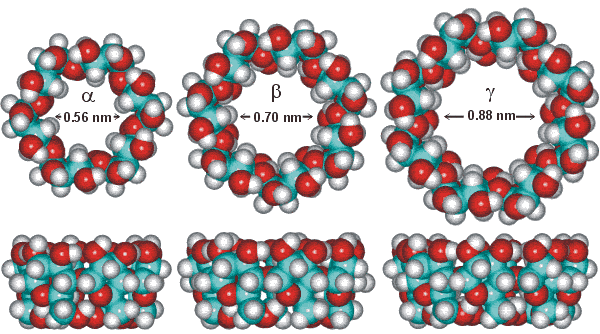
| Cyclodextrin | Mass |
Outer diameter, (nm) |
Cavity diameter (nm) |
Cavity volume, (mL/g) |
Hydrate H2O [915] |
|||
Inner rim |
Outer rim |
cavity |
external |
|||||
| α, (glucose)6 | 972 |
1.52 |
0.45 |
0.53 |
0.10 |
129.5 |
2.0 |
4.4 |
| β, (glucose)7 | 1134 |
1.66 |
0.60 |
0.65 |
0.14 |
18.4 |
6.0 |
3.6 |
| γ, (glucose)8 | 1296 |
1.77 |
0.75 |
0.85 |
0.20 |
249.2 |
8.8 |
5.4 |
Cyclodextrin rings are amphipathic with the wider rim displaying the 2- and 3-OH groups and the narrower rim displaying 6-OH group on its flexible arm. These hydrophilic groups are on the outside of the molecular cavity whereas the inner surface is hydrophobically lined with the ether-like anomeric oxygen atoms and the C3-H and C5-H hydrogen atoms. In aqueous solution, this hydrophobic cavity contains about 3 (α-DC), 7 (β-DC) or 9 (γ-DC) poorly held (but low entropy) and readily displaceable water molecules. This water in the cavities has low-density as the cavities are large enough to accommodate several more molecules. Thus, the otherwise hydrophilic cyclodextrin molecules may bind non-polar suitably-sized aliphatic and aromatic compounds such as aroma compounds and lipophilic drugs. They may bind in 1:1, 2:1 and 1:2 ratios dependent on the molecules involved (for example, two molecules of γ-cyclodextrin bind well to single C60-fullerene molecules [944]). The binding is driven by the enthalpic and entropic gain on the reduction in the hydrophobe-aqueous surface and the release of water molecules from the cavity to the bulk phase. Such binding also allows cyclodextrins to be used to increase the water solubility of hydrophobic compounds or minimize undesirable properties such as odor or taste in certain food additives. Cyclodextrin complexes are now widely used in the pharmaceutical, food and cosmetic and toiletry fields [919].
γ-Cyclodextrin is most flexible and readily hydrolyzed by α-amylases whereas α-cyclodextrin is most rigid and only very poorly hydrolyzed. The cyclodextrins, by themselves, are natural, non-toxic additives. The hydroxyl groups may be derivatized to modify the specificity, physical and chemical properties of the cyclodextrins. The 6-OH groups are most easily derivatized.
The low solubility of β-cyclodextrin, when compared to α- and γ-cyclodextrins presents a puzzle [915]. In a similar manner to the poor solubility of scyllo-inositol, it appears the stronger crystal structure (as with cellulose), due to better placed intramolecular hydrogen-bonding, together with a similarly better fit with the structure of water, and consequential low entropy of hydration, are responsible. Molecular dynamics shows a significant increase of the local water structure and a slower mobility of the surrounding water, with a reduction of ordered tetrahedral water molecules localized inside the hydrophobic cavity. Cyclodextrins, their derivatives, and complexes may self-assemble in water to form transient clusters or aggregates. Such clusters may dissociate upon shaking or dilution but the clustering may have significant effects on the physiochemical properties of cyclodextrins and their complexes including their ability to act as drug-delivery systems [3533]..
There is a growing interest in chemically modified cyclodextrins, such as hydroxypropyl‐β‐cyclodextrin and sulfobutyl ether‐β‐cyclodextrin, used in drug formulation, cosmetics, and the toiletry industries [3451]. The modifications are made to increase their water solubility and overcome any restrictions on their pharmaceutical use.
Larger cyclodextrins such as cyclomaltononaose (δ-cyclodextrin) and cyclomaltodecaose (e-cyclodextrin) are also found, if more expensive to produce, but as their ring size increases their stiffness diminishes, and the ring becomes slightly twisted, so reducing its binding capacity. Also, they become much more easily hydrolyzed by α-amylases. Even larger ring structures such as (glucose)14 and (glucose)26 have been crystallized. They have entirely different conformations involving flips and helices [918]. Smaller cyclodextrins such as cyclomaltotriose (CD3), cyclomaltotetraose (CD4), and cyclomaltopentaose (CD5) are also found [3538]
Interactive structures are available (Jmol).
a M. A. Villiers, Sur la fermentation de la fécule par l'action du ferment butyrique, Comptes Rendus, 112 (1891) 536-537, (in french).
Home | Site Index | Starch | Hydrocolloids | Polysaccharide hydration | hydrogen-bonding | LSBU | Top
This page was established in 2005 and last updated by Martin Chaplin on 1 June, 2019