
Yellowstone fumarole

![]() Water dimer and small clusters
Water dimer and small clusters
![]() Water vapor
Water vapor
![]() The Leidenfrost effect
The Leidenfrost effect
![]() Hexagonally-patterned microdroplets
Hexagonally-patterned microdroplets
Most of the water vapor in the air is derived by evaporation from the sea; particularly tropical seas. Surface temperature and wind speeds play key roles. The water vapor saturation over ice is less than over a water surface at the same temperature.
At sufficiently high-temperatures/low-pressures all particles in a gas phase obey the Ideal Gas Law.
PV = nRT
where P, V, T, and n are respectively the pressure (Pa), volume (m3), temperature (K), and the amount of the gas (mol) and R is the ideal gas constant (J ˣ mol-1 ˣ K-1). The Ideal Gas Law does not hold exactly at higher-pressures/lower-temperatures when the finite size of the particles and the interactions between particles have significant effects. The empirical equation below then fits better,
(P + n2a/V2) ˣ (V -nb) = nRT
where a and b have partial theoretical bases on the reduced pressure due tio the attractions of the molecules (a) and the size of the molecules (b), respectively. Both (a) and (b) may vary with the temperature and density.
Gaseous water is water vapor and one of the lightest of gasses. In science and engineering, the word 'steam' is also used for water vapor, but usually when above the boiling point of water. As commonly used in the English language, 'steam' also may mean the white cloud of fine liquid water droplets of condensed water vapor that is produced by a boiling kettle or at fumaroles (see right), for example.
Water vapor is transparent to 'visible' light but absorbs strongly in the infrared. It is the main absorber of the sunlight in the atmosphere; without it, the Earth would be in a permanent ice-age. The mechanisms through which water vapor in the lower troposphere (at temperatures up to around the triple point of water; ≈ 0.01 °C, ≈ 5 km) influences convection, circulation and the formation of clouds, have been reviewed [3139].
The 13 trillion tons of water in the atmosphere (≈ 0.33% by weight) is responsible for about 70% of all atmospheric absorption of radiation, mainly in the infrared region where water shows strong absorption. Atmospheric water contributes substantially to the greenhouse effect, more than twice that due to carbon dioxide, ensuring a warm habitable planet, but operates a negative feedback effect, due to cloud formation reflecting the sunlight away. Molecular rotations and vibrations are obtained using the spectroscopic data and summarized for all of water's isotopologues by IUPAC [3035].
As with other gasses, gaseous water is not an ideal gas, although it behaves nearly as an ideal gas at low densities, approximately obeying the relationship (the equation of state, EoS),
P = r ˣ R ˣ T
P = kB ˣ T/V
where P is the pressure ( Pa), r is the molar density (mol ˣ m-3 ), R is the gas constant ( J ˣ mol-1 ˣ K-1), T is the temperature (K), kB is the Boltzmann constant, and V is the volume (m3) of a mole of the molecules (mol ˣ NA).
At higher densities, corrections can be applied
P/rRT= 1 + Br + Cr2 + Dr3 +...
P = kB ˣ T ˣ (1/V + B2(T)/ V2 + ...
where B2(T) is negative at low temperature and positive at high temperatures. At B2(T) = 0, attractive and repulsive forces are in balance, as in an ideal gas [3853].
Variation of the second virial coefficient, from [3034]
![Variation of the second virial coefficient with temperature, from [3034] Variation of the second virial coefficient with temperature, from [3034]](images/virial.gif)
The second virial coefficient (B) depends on temperature. Except at high densities, the higher virial coefficients (C, D, etc.,) are usually ignored (i.e., they are put equal to zero). It can be seen right that the second virial coefficient increases considerably at lower temperatures [3034]. This indicates greater deviations from ideal behavior at lower temperatures which are partially due to dimer formation (shown blue right [3037]). The data for D20 is also shown as dotted lines with only limited data available for D20 second virial coefficient [3038].
hydrogen-bonding between water molecules occurs in the gas phase with, typically within the ambient atmosphere, over one water dimer forming for every thousand free water molecules rising to about one in twenty in steam. The dimer formation causes deviations from ideal gas behavior in gaseous water.
The thermodynamic properties of gaseous water above 100 °C, 101,325 Pa are described by The International Association for the Properties of Water and Steam (IAPWS). Experimental data for dilute gas phase heavy water (D2O) are available for viscosity and thermal conductivity and has been calculated for molecular diffusion [3036]. The thermodynamic properties of gaseous water below 100 °C, supersaturated (supercooled) steam at conditions occurring in steam turbines, has been studied by a number of theoretical and semi-theoretical methods [3507].
The maximum amount of water in the air, at 1 atm
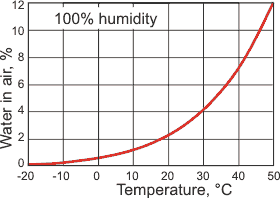
Water is present in the atmosphere in both liquid and gaseous forms. The maximum gaseous composition depends on the atmospheric temperature (see right). The average relative humidity of the atmosphere is about 75% at ground level reducing to about at 45% at 5000 m.
When we breathe out, we expire an aerosol of fine (nm - µm+ radius) water droplets plus water vapor. This aerosol has been detected by a water-cluster-detecting breath sensor developed for detecting drunk or drowsy drivers [1801].
The fugacity of water is described elsewhere.
[Back to Top ![]() ]
]
The Leidenfrost effect
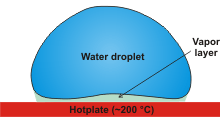
An interesting effect (the Leidenfrost effect, a [2291, 2771, 3649] see right), is that water droplets remain far longer on a hot plate just above 200 °C than if the hot plate was just above 100 °C. Once formed, the droplets move around the surface in a raoid motion. See [960] for an amusing scientific answer to how water boils. The Leidenfrost effect is not superheating but due to the formation of an insulating vapor layer keeping that liquid from boiling rapidly, with the water drops temperature remaining at about 90 °C when the plate is held at about 300 °C. The droplets rapid motion arises from an asymmetry in the vapor profile [3485]. If the hotplate surface is made superhydrophobic, then the droplets remain even at lower temperatures (down to 22 °C) [1926]. On heated, curved surfaces larger quantities of fluid, not just droplets, can be suspended [1957]. If placed in an electric field, Leidenfrost droplets jump from the hot surface higher and higher [2881]. The Leidenfrost effect has been used in nano-synthetic reactions to enable faster reaction rates. They may also be involved in sphere-in-cavity structures that have residual drag coefficients less than 10% of those of solid objects of the same dimensions [3118].
[Back to Top ![]() ]
]
Hexagonally-patterned microdroplets,
from [3331]
![Hexagonally-patterned microdroplets, from [3331] Hexagonally-patterned microdroplets, from [3331]](images/drop6.gif)
Self-organized hexagonally-patterned microdroplets (≈ 10 µm - 50 µm diameter) clusters have been more-recently found floating, as a single sheet, a dozen or so micrometers above locally-heated water (and other hydrogen-bonded liquids') surfaces [3331]. These flat levitating clusters of self-assembled ordered spherical droplets (see right) are formed by condensation of vapor above the locally (infrared or laser irradiated) heated water surfaces. They can be formed even at maximum surface temperatures as low as 27.6 °C. They are separated from each other and levitated above the liquid surface by about the droplet diameters. Their levitation is due to the dynamic balance between the evaporation and condensation balance for both water in the droplets and for the steam-air gas mixture around the droplets. Their organized array is due to the minimization of the entropy of the arrangement. The collapse of one droplet causes the collapse of all the droplets.
[Back to Top ![]() ]
]
a J. G. Leidenfrost, De aquae communis nonnullis qualitatibus tractatus, Ovenius, Duisburg, 1756. [Back]
Home | Site Index | Water phase diagram | LSBU | Top
This page was established in 2017 and last updated by Martin Chaplin on 29 January, 2020