
Ammonium, NH4+ Ammonia, NH3
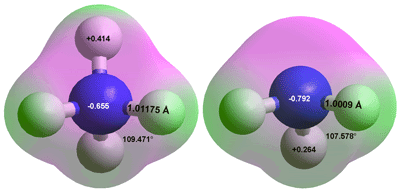
Ammonia (NH3) is a colorless flammable gas (boiling point, -33.34 °C; melting point, -77.73 °C, critical temperature 132.22 °C, critical pressure 11.345 MPa, critical density 234.3 kg ˣ m-3; [3685]) with a characteristic unpleasant and toxic odor. It is polar with dipole moment 1.5 D. In aqueous solution, it acts as a weak base and may be used as a general-purpose cleaner. Ammonia–water complexes, where NH3 molecules are are weakly hydrogen-bonded to surface water molecules are found on the surface of aqueous ammoniacal solutions [3687].
Ammonia is very soluble in water (it is the most soluble gas) where it reacts to form ammonium ions and hydroxide ions at appropriate pH. A saturated solution is highly corrosive and contains about 0.31 kg ammonia per kg solution at 25 °C, and has a density of about 0.88 g ˣ ml-1 (> 13 M). Solutions give off ammonia gas and concentrations cannot be considered as precise. These species are shown in the above right figure a where all the hydrogen atoms in both structures are equivalently positioned. The ammonia molecule may invert (like a wind-blown umbrella, see also the hydrogen ion). This inversion has an energy barrier of about 24 kJ ˣ mol-1 [3686] but in contrast to the H3O+ ion, the inversion is prevented by acceptor hydration at the nitrogen atom. The thermodynamic properties of ammonia-water mixtures have been reported [IAPWS, 3684].
H2O + NH3 ![]() OH- + NH4+ pKb (NH3) = 4.755 at 25 °C [3681]
OH- + NH4+ pKb (NH3) = 4.755 at 25 °C [3681]
H2O + NH4+![]() H3O+ + NH3 DH° = +51.92 kJ ˣ mol-1 at 25 °C [3680]
H3O+ + NH3 DH° = +51.92 kJ ˣ mol-1 at 25 °C [3680]
pKa (NH4+) = -Log10([H3O+] ˣ [NH3]/ [NH4+]) = 9.245 at 25 °C
pH = 9.245 + Log10([NH3]/ [NH4+]) at 25 °C
The relationship of pKa (NH4+) to temperature is pKa (NH4+) = 0.09018 + 2729.92/T where T is in Kelvin [3681].
Ammonium (NH4+) is a very weak acid, with an equilibrium constant (5.7 ˣ 10-10 M) approximately 70 times lower than physiological (buffered) hydrogen ion concentration (4.0 ˣ 10-8 M), so that when ammonia is produced in the body, it is immediately mostly converted (~98 %) to ammonium ions, but when added to pure unbuffered water it mostly remains as dissolved NH3.
Ammonia is a reducing agent and can be produced by the electro-synthesis of ammonia [3871]:
N2 + 6 H+ + 6 e- ![]() 2 NH3 E° = +0.55 V
2 NH3 E° = +0.55 V
N2 + 8 H+ + 6 e- ![]() 2 NH4+ E° = +0.27 V
2 NH4+ E° = +0.27 V
Ammonia may be oxidized 4 NH3 + 3 O2 ![]() 2 N2 + 6 H2O
2 N2 + 6 H2O
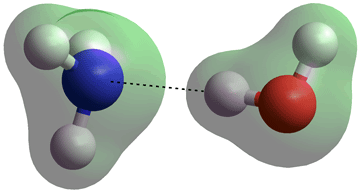
H3N···H-OH hydrogen bond in the gas phase
cis- and trans- H3N···H-OH hydrogen bonds
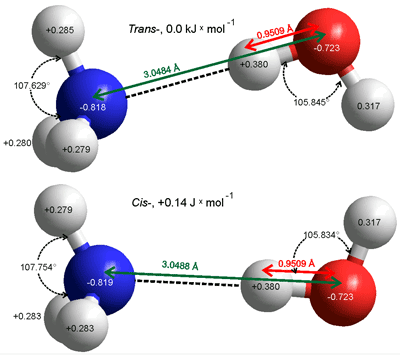
When attached to the water molecule, both the ammonia molecule and the water molecule lose some of their symmetry and the ammonia molecule cannot invert. The two very shallow energy minima cis- and trans- structures of the H2N-H ···OH2 hydrogen bond in the gas phase are given on the left. The energy difference (0.14 J ˣ mol-1) is not significant when compared with that of the hydrogen bond (25 kJ ˣ mol-1) and the thermal energy (2.5 kJ ˣ mol-1) leaving the H2O and NH3 molecules to freely rotate around the hydrogen bond. The (partially constrained) molecular parameters have also been determined with the CCSD(T)-F12A method and the VTZ-F12 basis sets with similar results. [3682]
H3N+-H···OH2 hydrogen bond in the gas phase
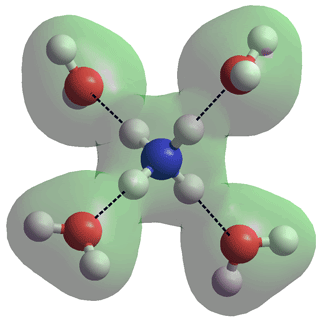
In aqueous solution, both NH3 and NH4+ are hydrated. NH4+ can form four strong, tetrahedrally-placed and long-lived, donor hydrogen bonds to H2O molecules [136] (see right, N-H, 1.01686 Å, N···O, 2.90274 Å). This structure is found to be much more stable than a cluster with an NH3 in the center and an H3O+ on the surface [194]. NH3 only forms one moerately-strong acceptor hydrogen bond (see the NH3 ···OH2 cis- trans- figure, above left) with the three much weaker donor H2N-H···OH2 (ultraweak) hydrogen bonds losing out to much stronger HO-H···OH2 interactions [3688]. In the magic ion NH4+(H2O)20, the NH4+ ion sits centrally in the water dodecahedron with the four hydrogen bonds from the central ammonium ion equivalent (see the connectivity map), so helping to explain the apparent increased structural stability of this ion relative to H3O+(H2O)20. Exchange of hydrogen bond partners by the central NH4+ explains its faster than expected rotation [855].
Note that a single H2O hydrogen-bonded NH4+ ion (H3N+-H···OH2) forms one of the strongest hydrogen bonds known at 92.5 kJ ˣ mol-1 [447]. In this structure, the hydrogen bond H3N+-H···OH2 shrinks by 0.15 Å (and the N-H covalent bond expands by 0.018 Å) between the tetra-coordinated and the single-coordinated NH4+, in line with the stronger bonding of the latter.
Under high pressures, such as occur in large planetary systems like Neptune and their moons such as Titan, Callisto, and Ganymede, mixtures of water and ammonia may form a complex phase diagram with many crystal forms [3683]. The multiplicity of these is not only due to same reasons as for the complexity of the water phase diagram but plus the additional complexity introduced by the multi-component-system present. A total of twelve mixed phases exist containing both NH3 and H2O molecules under various pressure–temperature conditions; ammonia monohydrate (NH3:H2O = 1:1, six phases), ammonia dihydrate (NH3:H2O, 1:2, four phases), and ammonia hemihydrate (NH3:H2O, 2:1, two phases) [3861].
[Back to Top ![]() ]
]
a The molecular structures on this page were calculated in the gas phase, using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. This is in line with other calculated structure factors given in this website (e.g., the values for the water molecule and its clusters). [Back]
Home | Site Index | Solubility of non-polar gases | Hydrophobic hydration | Hydro colloids | Saccharides hydration | Protein hydration | Nucleic acid hydration | Aqueous biphasic systems | Gas-liquid interface and nanobubbles | LSBU | Top
This page was established in 2019 and last updated by Martin Chaplin on 25 February, 2020