
Cytoskeleton in the human cell
recolorized from wellcomimages
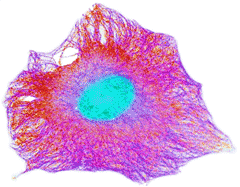
There is a dynamic coupling between intracellular water and active metabolic processes.
![]() Intracellular solutions contain more K+ ions
Intracellular solutions contain more K+ ions
![]() Membranes help create a tendency towards low-density water in cells
Membranes help create a tendency towards low-density water in cells
![]() The effect of intracellular protein on water structuring
The effect of intracellular protein on water structuring
![]() The importance of protein carboxylate groups
The importance of protein carboxylate groups
![]() The importance of protein mobility
The importance of protein mobility
![]() Cooperative conversion of the water structuring
Cooperative conversion of the water structuring
![]() Actin, tubulins and the intermediate filaments
Actin, tubulins and the intermediate filaments
"the molecular structure of water is the essence of all life"
Dr. Albert Szent-Gyorgy, Nobel Prize winner.
Intracellular water is the water inside cells that bathes all the necessary biological molecules including the proteins and nucleic acids. a The interior of the mammalian cell is a very crowded environment containing ≈ 250 mg ˣ mL-1 protein, ≈ 110 mg ˣ mL-1 RNA, ≈ 103 m2 ˣ L-1 cytoskeleton: surface, ≈ 250 mM ions, and many other solutes. Consequently, the internal osmotic pressure is high and the water activity is low. Crowding affects molecular diffusion conformation and dynamics and kinetics [3426], as well as the properties of the aqueous environment [3175]; with viscosity ~106 times higher than bulk water and diffusion ~106 times lower than bulk water. Intracellular water is necessarily different from extracellular water [3006]. Cells contain differing amounts of water; dependent more on their purpose (and hence expressed proteins) rather than their source organism (for example, the % water contents of red blood cells are about 64% whether from man or dog whereas frog heart contains 80% but a frog egg only 49% water) [762]. Intracellular water is in a more ordered state (coherent. [3714]) than pure water, as demonstrated by its significantly shorter (NMR) spin-lattice and spin-spin relaxation times [2321]. Very little of this water can be considered as solely a medium [2753] g but is a metabolic reactant, product, catalyst, chaperone, messenger, and controller (see for example, [1194]). Intracellular water cannot be pressed out of the cell even at 100 MPa and is likely to consist of organized nanoscale water channels rather than a homogeneous gel-like cytoplasm [3633]; the compessed water preferring to be absorbed into the protein structures.
Intracellular water is responsible for the conformations and functions of all biomolecules through direct interaction with their hydration shells [2845, 2921]. However and importantly the water's structuring may also be controlled by some cellular constituents. Cellular macromolecules ‘‘dance’’ to the tune of intracellular water and, to a more restricted extent, vice versa. Water forms an integral part of biomolecular structures and functions [2949]. The water is essential for biomolecular recognition [1787] and orchestrates the cell machinery [1785]. The complexity and organization within the cytoplasm are expressed in a
comprehensive review of intracellular water [1191] and an interesting current review has considered the versatility and adaptability of intracellular water in engaging in a wide range of cellular biochemistry [1359]. A key feature of intracellular water is its ability to convert between high and low-density states [1364] (as exemplified by the ES![]() CS equilibrium). Such changes have been linked to the state of autothixotropy [1898]. The extent of intracellular hydrogen bonding can be determined using an amphiphilic fluorescent nanodot. This shows imaging time-dependent contrasts due to the different hydrogen bonding extents of the intracellular water. The nanodot’s amphiphilic nature in different local hydrogen-bonding environment produces contrast images in lifetime and directly monitors the extent of the hydrogen-bonding networks [3512].
CS equilibrium). Such changes have been linked to the state of autothixotropy [1898]. The extent of intracellular hydrogen bonding can be determined using an amphiphilic fluorescent nanodot. This shows imaging time-dependent contrasts due to the different hydrogen bonding extents of the intracellular water. The nanodot’s amphiphilic nature in different local hydrogen-bonding environment produces contrast images in lifetime and directly monitors the extent of the hydrogen-bonding networks [3512].
An increase in cell size, following cell membrane depolarization, occurs together with a reduction in water's diffusivity, indicating the very different characteristics that intracellular water appears to possess when compared to extracellular water. b Experimentally, this area presents a difficult problem as the properties and functioning of the cell in vivo cannot be readily determined from the sum of its parts in vitro; indeed the commonly practiced in vitro experiments on the homogenized and dilute contents of dead cells may be entirely misleading. For examples, CO2 production in metabolizing cells may give rise to complex changes in water movement and osmotic pressure changes as neutral CO2 diffuses readily but ions formed from it on hydration do not [1137], and actively metabolizing cells may be producing a significant amount of their intracellular water [1139]. Study of the live cell is fraught with difficulty as most procedures may alter the in vivo conditions, although dynamic phase microscopy shows promise [1332]. The structure and hydrogen-bonding of intracellular water are central to any theory concerning intracellular cell dynamics with intracellular protein dynamics and internal protein fluctuations both slaved to the water dynamics [1856]. There are two theories concerning intracellular water that have greatly influenced current thinking concerning the complex roles of intracellular water. These are the 'polarized multilayer theory' of Gilbert Ling [634, 2807], and the 'gel-sol transition' of Gerald Pollack [351, 635]. On this page, the combination of aspartic and glutamic acid ion-pairing with K+ ions, changes in the mobility of key proteins and the natural low-density clustering within the intracellular water are all shown to contribute towards intracellular metabolic transitions and information transfer. Both prior theories are accommodated within this hypothesis [1093].
Water in cancerous breast tissue is markedly different from that in the healthy tissue, in having more water plus a greater concentration of free hydroxyl groups [2280]. The intracellular water lifetime may be considered as a hallmark of cancer, with cancerous cells showing increased cell volumes as cells undergo proliferation [3495].
[Back to Top ![]() ]
]
The cytoskeleton including a microtubule (largest), an intermediate
filament (knobby one) and two actin filaments (smallest)
Illustration by David S. Goodsell, the Scripps Research Institute
The different characteristics of the intracellular and extracellular environments manifest themselves particularly in terms of restricted diffusion (see cartoon representing the crowded cytoplasm, right) and a high concentration of chaotropic inorganic ions and kosmotropic other solutes within the cells. Note that both chaotropic inorganic ions and kosmotropic other solutes encourage low-density water structuring. The difference in concentration of the ions is particularly apparent between Na+ and K+ (see below); Na+ ions (2.38 À distant from water O-atoms. [3868] ) creating more broken hydrogen-bonding and preferring a high aqueous density whereas K+ ions (2.76 À distant from water O-atoms [3868]) prefer a low-density aqueous environment [3796] . A 1000-fold preference for K+ over Na+ has been found in a halophilic organism without any energy expenditure but with a highly reduced intracellular water mobility [817]. As explained in the discussion of the Hofmeister effect, and shown by the apparently negative ionic volumes (that is, addition of the ions reduces the volume of the water, see below), the interactions between water and Na+ are stronger than those between water molecules, which in turn are stronger than those between water and K+ ions. Thus, water may be stripped more easily from the K+ ions and K+ maintains the solubility of cell components in which carboxylate and phosphate are the principal anions [3796] . These comparisons may be explained by the differences in surface charge density. The interaction strength is reflected in that the distances between the Na+ ions and water are shorter than between pairs of water molecules which are shorter than those between K+ ions and water. Ca2+ ions have even stronger destructive effects on the hydrogen-bonding than Na+ ions. Clearly, K+ ions are preferred within the intracellular environment.
Ion |
Ionic radius |
Surface charge density |
Molar ionic volume |
Intra-cellular |
Extra-cellular |
Water preference |
|---|---|---|---|---|---|---|
Ca2+ |
100 pm |
2.11 |
-28.9 cm3 |
0.1 μM |
2.5 mM |
High-density |
Na+ |
102 pm |
1.00 |
-6.7 cm3 |
10 mM |
150 mM |
High-density |
K+ |
138 pm |
0.56 |
+3.5 cm3 |
159 mM |
4 mM |
Low-density |
Although somewhat contentious, it has been reported
that the cellular membrane ion-pumps
cannot produce these large differences in ionic composition
in the absence of other mechanisms. The (ATP) energy
required appears to exceed the energy that is available to
the cell, and the gradients are maintained in the absence of
intact membranes and the absence of active energy (that is, ATP) production [634, 635].
Also, in contrast to that written in several undergraduate
textbooks, many studies show that cells do not need an intact
membrane to function [635]. Instead,
the intracellular water tends towards a low-density structuring
due to the kosmotropic character of the majority of the solutes,
the confined space within the cell stretching the hydrogen-bonded
water and the extensive surface effects of the membranes [1094].
The ions partition according to their preferred aqueous environment;
in particular, the K+ ions partition into the cells.
Ion pumps must thus be present for other (perhaps fail-safe)
purposes, such as speeding up the partition process after
metabolically linked changes in ionic concentration. [Back to Top ![]() ]
]
Phosphatidylethanolamine
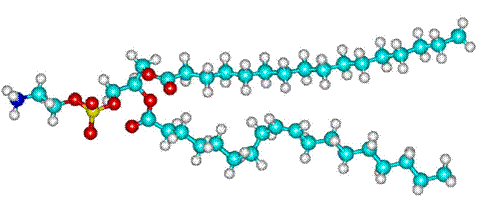
Membrane lipids contain hydrophilic kosmotropic groups such
as the phosphatidylethanolamine, shown opposite, which encourages
the lower density water structuring inside cells. This is particularly
relevant as there is extensive membrane interfacial water within
cells, e.g., liver cells contain ≈ 100000 μm2 membrane surface area. The aqueous interface next to the membranes forms a functional unit linking the membrane to the water-soluble proteins of the cytosol, facilitating the rapid exchange of structural, dynamic and physiological information [1553]. [Back to Top ![]() ]
]
The degree of density lowering inside cells is determined by the solutes, their concentration and the conformations and state of motion of the proteins; mobile proteins creating more disorder in the clustering compared with more static proteins. Many intracellular proteins are globular, so retaining rotational entropy notwithstanding the crowded environment. Water has conflicting effects in the mixed environments around proteins. Weak hydrogen-bonding between the protein and water molecules allows greater flexibility. Strong hydrogen-bonding endows the protein with greater stability and solubility. There is an ordered structure in the first shell around the protein, with both hydrophobic clathrate-like and hydrogen-bonded water molecules each helping the other to optimize water's hydrogen-bonding network. Changes in the proteins' conformations may result in water influx into the cell or efflux from the cell [949] as well as density changes. Protein carboxylate groups are generally surrounded by strongly hydrogen-bonded water whereas the water surrounding basic chaotropic groups (arginine, histidine, and lysine) tends towards a clathrate structuring. Clathrate formation over hydrophobic areas maximizes non-bonded interactions without loss of hydrogen bonds whereas carboxylate groups usually only fit a collapsed water structure creating a reactive fluid zone. The diffusional movement of the proteins will cause changes in the water structuring outside the first hydration shell.
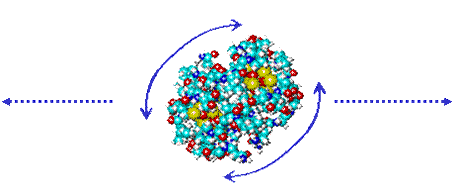
Translational diffusion involves breaking water-water links at a distance from surface whereas rotational diffusion involves breaking close water-water and protein-water links [640]. The surface area for translation and rotation is the same, but the velocity differential is constant for all radial distances (r) for translation but varies with r2 with rotation.
At the breaking surface, half the hydrogen bonds are ruptured, therefore
creating a zone of higher density water (as explained
elsewhere). Protein rotation thus creates a relatively
close and surrounding high-density water zone with many broken
hydrogen bonds, and as shown by some NMR studies. The volume
of this interfacial region of perturbed water around the proteins
is comparable to the volumes of the proteins. [Back to Top ![]() ]
]
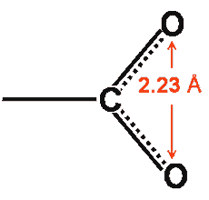
The side-chain carboxylate groups (that is, aspartic and glutamic acids) in proteins possess high dipole moments and contain two oxygen atoms that are nearer to each other (≈ 2.23 Å) than occurs between water molecules in bulk liquid water (≈ 2.82 Å). These carboxylate oxygen atoms preferably hydrogen bond ≈ 4 [1169] to ≈ 6 [1781] water molecules which account for about 40% of the hydration free energy with the remainder mostly derived from outer aqueous shells [1781 a]. f This hydrogen-bonding causes a localized high-density water clustering due to the closeness of the water molecules as they gather around the carboxylate groups [1063]. Binding of water to the two oxygen atoms is anti-cooperative, and instantaneously the oxygen atoms and C-O bonds are non-identical [1587].
Such hydrogen-bonding induces a more negative charge on the carboxyl oxygen atoms, increasing the group's dipole and this leads to an increase in the carboxylate pKa, as is shown below.
Carboxylate group dipoles versus their pKa
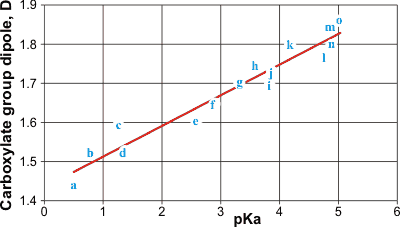
The atomic charges and geometry of related carboxylate were determined using ab initio molecular dynamics with the 6-31G** basis set in Hyperchem. The dipoles of the carboxylate groups were calculated in Debye units. A linear trend can be seen between the carboxylate group dipole and the pKa of the acids: a, trifluoroacetate; b, trichloroacetate; c, dichloroacetate; d, difluoroacetate; e, fluoroacetate; f, chloroacetate; g, thioglycollate; h, thiolactate; i, glycollate; j, lactate; k, 3-thiopropanoate; l, acetate; m, 2-methylpropanoate; n, propanoate; o, 2,2’-dimethylpropanoate. Most of the acidity constants were obtained from [1142].
Carboxylate groups pKa and oxygen charge
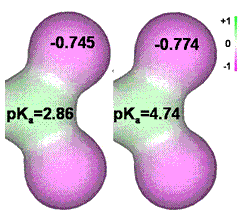
If the charges on the carboxylate oxygen groups are reduced, by neighboring electron-withdrawing substituent groups or imposed negative electric field, the pKa is reduced. Note that the charge on the oxygen atoms lies between that on the ionic kosmotrope sulfate (–0.87; with hydrogen bonds to water) and the ionic chaotrope perchlorate (–0.71; preferring to be surrounded by clathrate water). Thus, only a 5% reduction in the negative charge on the carboxylate oxygen atoms is necessary to switch from kosmotropic to chaotropic behavior. It is found that Na+ ions prefer binding to the weaker carboxylate groups (pKa > 4.5) whereas K+ ions prefer the stronger acids (pKa < 3.5) [634].
It should be noted that, in the absence of an aqueous solution, Na+ ions bind with a covalent character to the glutamate carboxylate groups whereas K+ ions do not [3070]. [Back to Top ![]() ]
]
Part of the F-actin structure
structure from Holmes KC and Eschenburg S
Actin is a highly conserved and widespread eukaryotic protein (42-43 kDa) e responsible for many functions in cells. Non-muscle cells contain 5-10% (of all protein) actin whereas muscle cells contain about 20%. All actin molecules contain a conserved acidic N-terminus with several neighboring aspartic or glutamic acids (emphasized right) and a post-translation acetylated terminal amino group (for example, N-Ace-Asp-Glu-Asp-Glu in rabbit muscle α-actin). When actin forms a filament structure this highly charged grouping is placed on the outer exposed edge of the helix, where it may be additionally used as a binding site for other proteins, such as myosin. The positionings of the acidic N-terminal antennae are shown in red in the cartoon below. Tubulin and the intermediate filaments are further structural proteins that form immobile structures with ordered hydration within cells, possessing conserved acidic groupings that serve similar functions. e
Cartoon of the semi-flexible actin filament, showing the positioning of the acidic N-termini (shown red)

The structural transitions of F-actin upon ATP hydrolysis at near-atomic resolution have been revealed by cryo-EM, demonstrating how ATP hydrolysis links to F-actin’s conformational dynamics and protein interaction [3295]. Actin is converted between a freely rotating molecule (G-actin; about 4 - 6 nm diameter) and a static right-handed double helical protein filament (F-actin; up to several microns in length) by ATP; a process involving the conversion of an α-helix to a β-turn in one of the structural domains [636]. Each molecule of the freely rotating G-actin can influence a large volume of water within its effective radius of gyration, causing a reduction in the intracellular low-density aqueous clustering. Filamentous actin (F-actin) is a much more ordered structure so creating more order in its surrounding water. Protein fibers trap water, which has decreased entropy. To attempt to keep the water activity constant, therefore, the water has to form bonds with a more negative enthalpy. This results in stronger bonds, causing greater structuring and lower density. Also, the enclosure of the water involves capillary action forming 'stretched' confined water that is much more highly structured (hence lower density) than the bulk water.
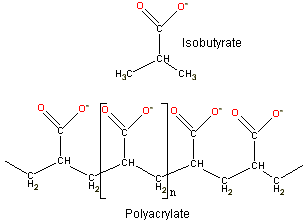
As overlapping fields from nearby groups enhance counter-ion association, F-actin's extensive negatively charged N-terminus will cause the partitioning of cations into its vicinity, in a similar manner to that well known from immobilized enzyme studies. This partitioning effect was initially shown by Kern [638] who established that the activity of Na+ with isobutyric acid is >0.9 whereas similarly with polyacrylate it is <0.32, thus proving the greater partitioning of cations within the microenvironment of the multiply-charged polymer.
Na+ and K+ ions behave differently when close to the carboxylate groups; K+ ions have a preference for forming ion-pairs whereas Na+ ions form solvent separated pairings [637, 2035]. This is due to the Na+ ions holding on to their water strongly with the inner shell polarized water molecules being ideally situated for forming strong hydrogen bonds to the carboxylate oxygen atoms. The K+ ions prefer an inner shell clathrate water structuring that is reinforced by its direct ion-pairing to the carboxylate group. Also, Na+ ions cannot take part in clathrate structuring whereas K+ prefers this environment [641]. As the distance between the neighboring amino acid carboxylate groups (about 7.3 Å) is about the same as the diameter of clathrate water clusters (about 7.6 Å), extensive ion-pairing of the adjacent carboxylate groups may be accommodated by neighboring clathrate clustering. K+ RCO2- pairs move from solvent separated to ion-pair with the reduction in the pKa. K+ ions prefer strong acids (that is, low pKa) whereas Na+ prefers weak acids (that is, higher pKa). Direct ion-pair association discourages aqueous hydrogen-bonding to the carboxylate oxygen atoms but encourages clathrate formation surrounding the ion-paired carboxylate group.
Note that the association of K+ with proteins' aspartate and glutamate groups is the central theme of Ling's fixed charge hypothesis [634]. He presented as evidence for the molecular mechanism of the association including (1) the low intracellular electrical conductance, (2) the strongly reduced mobility of intracellular K+ ions, (3) the altered X-ray absorption fine edge structure, (4) the widely different activity coefficients of K+ ions in different cells, (5) K+ ion absorption shows one to one stoichiometric absorption to the carboxylate groups, and (6) identification of K+ ion absorption sites as aspartate and glutamate side chains.
Equilibria involving the static polymerized protein
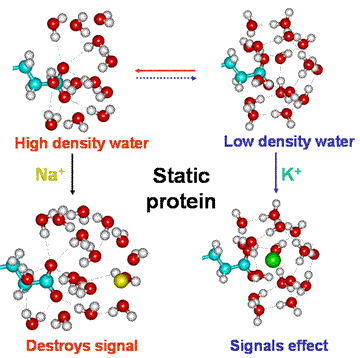
Under conditions when the carboxylate groups possess high pKa, both K+ and Na+ form solvent separated species when partitioned into the carboxylate environment. This gives a preference for Na+ and high-density water. However, rotation of the protein will tend to sweep such ions, and their associated water, away. If the protein stops rotating, Na+ ions tend to destroy any low-density structuring around carboxylate groups of the protein. However, usually the intracellular Na+ ion concentration is far lower than that of K+ ions (see above). At low pKa, K+ ion-pairs to carboxylate groups, particularly when partitioned into their microenvironment due to the presence of several contiguous acidic amino acids.
This hypothesis is compatible with a recent view that the water on either side of a membrane is different and this encourages the differences in K+ and Na+ concentrations [3006].
Equilibria involving the freely rotating protein
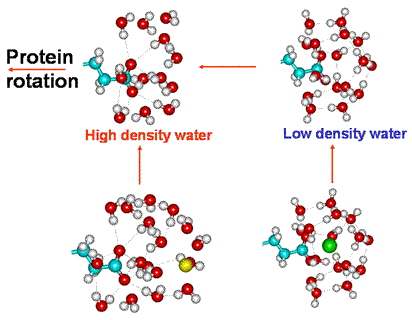
When ion-paired the hydrogen-bonding to the carboxylate oxygen atoms is prevented, and a surrounding clathrate structuring is preferred. The clathrate clustering can signal their state to other neighboring carboxylate groups (see later).
When the static protein filaments convert to freely diffusing
proteins, the ions are swept away due to the multiple contacts
with surrounding water molecules and their inability to keep
up with the rotating protein at increasing radial distances.
This results in the conversion of any localized clathrate
water structuring to high-density water involving hydrogen-bonded
carboxylate groups. [Back to Top ![]() ]
]
Binding of K+ by the carboxylate groups lowers the ionic strength of the intracellular solution. As this ionic strength decreases, the pKa of phosphate groups increases resulting in the conversion of the more kosmotropic intracellular doubly-charged HPO42- ions to the singly-charged more chaotropic H2PO4- ions. d All intracellular phosphate entities (about 100 milli-equivalents L-1) will behave similarly, so increasing the tendency towards the low-density water. Thus the cooperative effects of the change between static filament formation and freely-diffusional protein can be summarized as below.
Cooperative changes creating low-density water when actin is polymerized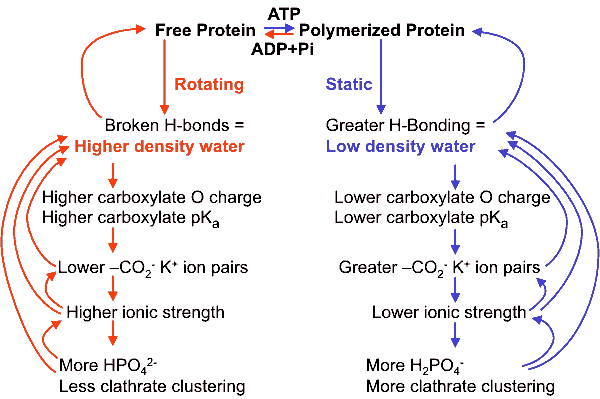
Further support for this process is given by F-actin becoming more static in the presence of about 100 mM K+ [642].
Cartoon showing the cooperative buildup of low-density clustering around
K+ on-paired carboxylate groups in proteins

Only the oxygen atoms of the water network are shown above.
The diameter of the cylindrical water cluster appears to be less than 3% different from that invoked to explain the hydrophobic effect between flat hydrophobic surfaces and extending over some tens of nanometers [1316], indicating that the same clustering may be involved in both cases.
The cooperative nature of this signaling explains the signal amplification seen in the conduction of impulses along the microtubules and actin [1434], e and perhaps linking to other parts of the cytoskeleton e [1307]. The importance of this cannot be overstated as microtubules are implicated in information processing and memory [1307]. The structuring of water may, therefore, be critical for the conscious mind [1337].
Although the clustering (above right) involves a significant drop in entropy (increase in coherence) , this is compensated by a more-negative enthalpy due to the stronger bonding of the fully-tetrahedral hydrogen-bonded structure. The phase transition between ordered and disordered water has been regarded as a mechanism to release energy for biological work rather than the membrane (pump) theory [1960]. It is consistent with Ling's association-induction polarized multilayer model [634], c as can be seen from the net dipoles emanating from the clathrate arrangement, but offers a more realistic explanation. The initial icosahedral size (3 nm diameter) also equals the water domain size proposed by Watterson [546]. Extensive (1.4 nm) long-lived (> 300 ps) aqueous dipole arrangements between biomolecules have been found in molecular dynamics studies [329]. Further support for this model is given by several studies concerning different types of intracellular water, such as "normal" bulk and "abnormal" osmotically inactive interfacial water [639]. Also, the water in the extreme halophile Haloarcula marismortui, which contains unusually large amounts of bound intracellular K+ ions, shows particularly slow translational diffusion [1253].
Idealized tetrahedral clustering caused by neighboring K+ carboxylate ion pairs
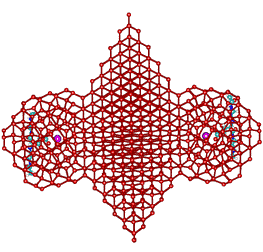
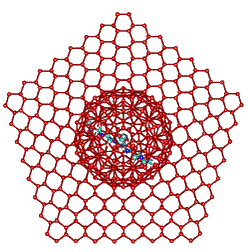
These orthogonal diagrams show the maximum limit of fully
tetrahedral water clustering due to the cooperative clustering
(see above) from two K+-carboxylate ion-pairs.
This fully tetrahedral structuring, which resembles a mixture
of hexagonal and cubic ice structures but with 5-fold symmetry
(such that it cannot be easily frozen), is idealized in the
cartoons. An interactive structure is given (Jmol).
Extension of the clathrate network and its associated low-density water enables K+ ion binding to all aspartic and glutamic acid groups, not just the key ones within the extensive acidic centers. Thus, the gel-sol transition of Pollack [635] is interpreted as the conversion to low-density water clustering (the gel state) due to clathrate clustering around K+-carboxylate ion-pairs. The changes in the functional state of the cell and its organisms has been shown to be reflected in the changes in the cell's refractivity [1997]. Certainly, the stiffness of the cytoplasm depends on the structure of the water as shown by changing the H2O water to D2O heavy water when the intracellular relaxation frequency reduces by between two and four orders of magnitude depending on the timescale [1856].
In the presence of raised levels of Na+ or Ca2+ ions, as occasionally occurs during some cell functions, these ions will replace some of the bound K+ ions. These newly-formed solvent-separated Na+ or Ca2+ ion-pairings destroy the low-density clathrate structures and initiate a cooperative conversion of the associated water towards a denser structuring.
In conclusion, the information transfer within the cell involves the following:
a Philosophical, historical, and scientific views of water in living systems have been reviewed [1011] [Back]
b The cytoplasm of cells is described in [892]. Nuclear magnetic resonance signal widths are much broader inside cells, showing that intracellular water is far more structured than extracellular or pure water; for example, see [884]. For a review of the relationship between the intracellular water and the cytoskeleton see [880]). It is noteworthy that the amount of 'free' versus 'bound' water [969], the concentration of K+ ions, and the cytoskeleton are all intimately linked together by the differences between normal and cancer cells [1333]. [Back]
c The polarized multilayer theory relies on the hypothesis that static proteins in the cell change their secondary structure from compact to extended forms, so that the newly formed extensive lengths of polypeptide 'polarize' the water using their amide (N-H and C=O) backbone. Although there is support for the existence of polarized multilayers of water [634], there is no experimental support for this explanation for their presence. Indeed, most experimental data is contra-indicative, resulting in only minority support from scientists in this area. [Back]
d The chaotropic/kosmotropic nature of these ions has been confirmed by FTIR [1072]. [Back]
Eukaryotic cells are dynamic structures, able to move, and divide. Such activities are achieved through the use of cytoskeletal filaments (actin, tubulins and intermediate filaments [3051], see cartoon above) and molecular motors (such as myosin) within the cell. The filaments lengthen, shorten and cross-link, regulated by other proteins. The actin filament is a helical ribbon formed of two parallel helical strands that each comprise a linear strand of small G-actin proteins (42 kDa) molecules with uniform orientation along the ribbon axis. The ribbon has a width of ∼8 nm, a thickness of ∼5 nm, and a helical repeat of 36 nm. An analysis of the known actin structures from the UniProt Knowledgebase showed the 32 complete α-actin structures recorded from fish, birds, snakes, and mammals all to have the conserved acidic N-terminal structure Acetyl- D/E- D/E- D/E- D/E- T/S/V- T- A- L- V- C- D- N- G- S- G- L- V- K- A- G- F- A- G- D- D- A- P- R- A- V- F- P- S- I- V- G- R- P- R- H- Q- G- V- M- V- G- M- G and the 70 complete β- and γ-actin structures recorded, from a similar range of species, to all have the conserved and closely related N-terminal structures of Acetyl- D/E- D/E- D/E- I/V/T- A/T- A/S- L- V- V/I/C- D/A- N- G- S- G- M/L- C- K- A- G- F- A/G- G/R- D- D/G- A- P- R- A- V/A- F- P- S- I- V- G- R- P- R- H- Q- G- V- M- V- G/R- M- G with even the variations limited to one or a very few molecules.
Tubulin showing the α/β units with acidic C-termini (red)

Eukaryotic tubulin is another structural protein that forms immobile structures with ordered hydration within cells [689]. It forms fat hollow and stiff microtubules (≈ 25 nm in diameter, containing low-density water) making tracks through the intracellular space for the movement of organelles. These tubular structures are built up from pairs of similar subunits (α and β), each of about 450 amino acids [1140]. Tubulins have a GTP binding domain near their N-terminal and two separate β-sheets each surrounded by α-helices. The microtubules are formed from head to tail arrangement of α/ β dimers with the beta-subunit GTP at the open end. Only this GTP is hydrolyzed following polymerization. Outside the molecular core, two exterior antiparallel α-helices lead to highly acidic C-termini, which are thus situated on the outside of the microtubule and away from the tubulin surface [1306]. The α-tubulin structure is highly conserved with C-terminal consensus sequence - A- R- E- D- M- A- A- L- E- K- D- Y- E- E- V- G- V- D- S- V- followed by an extremely variable but highly acidic C- terminus exemplified by the human α- tubulin (type ‘ubiquitous’) structure - E- G- E- G- E- E- E- G- E- E- COOH. The β- tubulin structure is also highly conserved with C- terminal consensus sequence - A- E- S- N- M- N- D- L- V- S- E- Y- Q- Q- Y- Q- D- A- T- A- followed by an extremely variable highly acidic C- terminus exemplified by the human β- tubulin (type ‘4Q’) structure - E- E- E- E- D- E- E- Y- A- E- E- E- V- COOH. As with the actins, these examples indicate that very strongly conserved structures exist for tubulins except for at an end (here the C-terminus) where glutamic acid and aspartic acid occur apparently interchangeably and in variable but always very acidic sub-structures. An improper functioning of tubulin has been linked with intracellular water clustering in the origin of cancer [1901].
Vimentim tetramer fragment, a type III intermediate filament

Intermediate filaments are fibrous, flexible and elastic proteins formed mainly from coiled coils (‘ropes’, ≈ 11 nm diameter) of multi-stranded acidic α-helices [1141]. There are some families of associated proteins such as the nuclear lamins, sarcomere desmins, neuron neurofilaments and epithelial cytokeratins. In addition, the vimentins (see right) generally support the organelles and maintain cellular integrity. All these proteins possess α-helical central ‘rod’ domains (≈ 48 nm long) that contain about 310 amino acids plus more variable end domains, while some acidic groups form salt links with basic groups in other strands to build up and lengthen the resulting ‘elastic rope’ structure, there exists an excess of acidic groups on the surface of the filaments. Within the α-helices are acidic bulges (formed from single π-helical loops) containing clusters of three glutamic acids within four residues and creating flexible ‘linker’ regions. As different strands come together to form the fibrous ‘ropes’ these acidic clusters are grouped together with contributing acidic clusters from about 20 helices on the surface of the filaments. [Back]
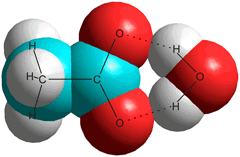
f Time-resolved infrared pump-prime spectroscopy of acetate in D2O suggests the bridge structure (right) as the most stable (single-hydrated) structure [1804], but other water molecules are also involved.
The optimal configurations, energy parameters, and normal vibrational frequencies of the hydrates of the acetic acid and acetate molecular hydrates have been calculated using density functional theory. The preferred structures CH3COOH⋅(H2O)2, CH3COOH⋅(H2O)8, CH3CO2-⋅(H2O)2, CH3CO2-⋅(H2O)2, CH3CO2-⋅(H2O)6, and CH3CO2-⋅(H2O)16 [3151]. [Back]
g Ultrafast vibrational spectroscopy and dielectric-relaxation spectroscopy have shown somewhat higheramounts of bulk-like water in Escherichia coli and Saccharomyces cerevisiae [3494]. [Back]
[Back to Top ![]() ]
]
Home | Site Index | Protein hydration | Nucleic acid hydration | Aqueous biphasic systems | LSBU | Top
This page was established in 2004 and last updated by Martin Chaplin on 7 March, 2020